9 | Medical Evaluation of the Surgical Patient |
Cardiovascular and pulmonary complications continue to account for major morbidity and mortality in patients undergoing noncardiac surgery. Emerging evidence-based practices dictate that the internist should perform an individualized evaluation of the surgical patient to provide an accurate preoperative risk assessment and stratification that will guide optimal perioperative risk-reduction strategies. This chapter reviews cardiovascular and pulmonary preoperative risk assessment, targeting intermediate- and high-risk patients with the goal of improving outcome. It also reviews perioperative management and prophylaxis of diabetes mellitus, endocarditis, and venous thromboembolism.
EVALUATION OF INTERMEDIATE- AND HIGH-RISK PATIENTS
Simple, standardized preoperative screening questionnaires, such as the one shown in Table 9-1, have been developed for the purpose of identifying patients at intermediate or high risk who may benefit from a more detailed clinical evaluation. Evaluation of such patients for surgery should always begin with a thorough history and physical examination and with a 12-lead resting electrocardiogram (ECG), in accordance with the American College of Cardiology/American Heart Association (ACC/AHA) guidelines. The history should focus on symptoms of occult cardiac or pulmonary disease. The urgency of the surgery should be determined, as true emergency procedures are associated with unavoidably higher morbidity and mortality risk. Preoperative laboratory testing should be carried out only for specific clinical conditions, as noted during clinical examination. Thus, healthy patients of any age who are undergoing elective surgical procedures without coexisting medical conditions should not require any testing unless the degree of surgical stress may result in unusual changes from the baseline state.
STANDARDIZED PREOPERATIVE QUESTIONNAIREa |
PREOPERATIVE CARDIAC RISK ASSESSMENT
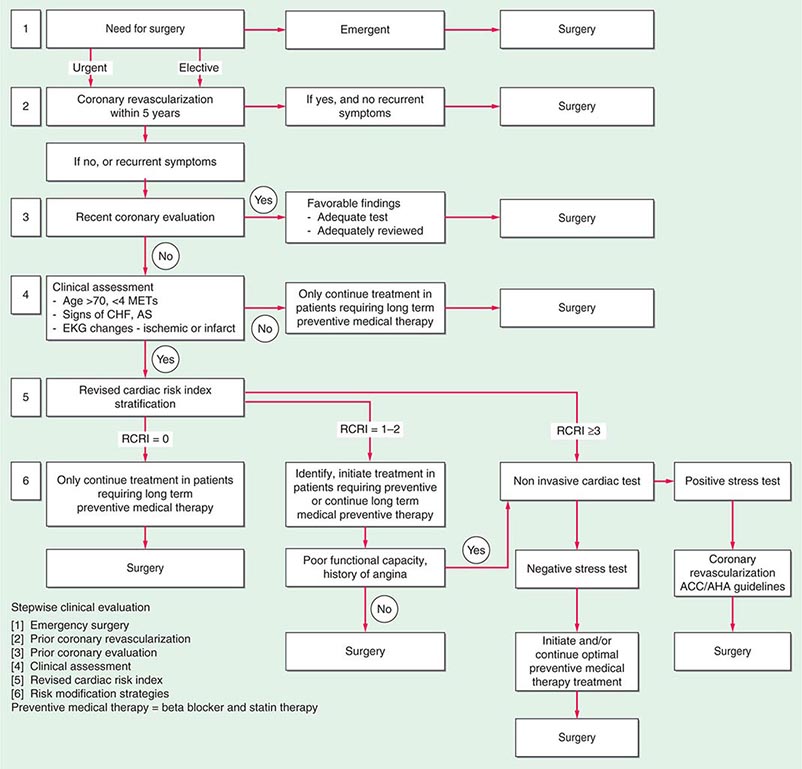
A stepwise approach to cardiac risk assessment and stratification in patients undergoing noncardiac surgery is illustrated in Fig. 9-1. Assessment of exercise tolerance in the prediction of in-hospital perioperative risk is most helpful in patients who self-report worsening exercise-induced cardiopulmonary symptoms; those who may benefit from noninvasive or invasive cardiac testing regardless of a scheduled surgical procedure; and those with known coronary artery disease (CAD) or with multiple risk factors who are able to exercise. For predicting perioperative events, poor exercise tolerance has been defined as the inability to walk four blocks or climb two flights of stairs at a normal pace or to meet a metabolic equivalent (MET) level of 4 (e.g., carrying objects of 15–20 lb or playing golf or doubles tennis) because of the development of dyspnea, angina, or excessive fatigue (Table 9-2).
ASSESSMENT OF CARDIAC RISK BY FUNCTIONAL STATUS |

FIGURE 9-1 Composite algorithm for cardiac risk assessment and stratification in patients undergoing noncardiac surgery. Stepwise clinical evaluation: [1] emergency surgery; [2] prior coronary revascularization; [3] prior coronary evaluation; [4] clinical assessment; [5] RCRI; [6] risk modification strategies. Preventive medical therapy = beta blocker and statin therapy. RCRI, revised cardiac risk index. (Adapted from LA Fleisher et al: Circulation 116:1971, 2007, with permission.)
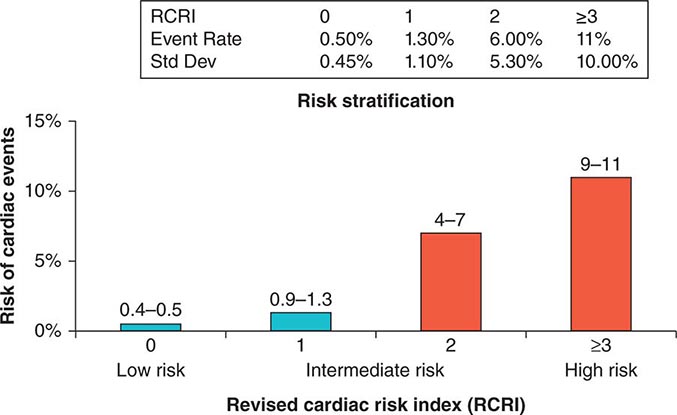
Previous studies have compared several cardiac risk indices. The American College of Surgeons’ National Surgical Quality Improvement Program prospective database has identified five predictors of perioperative myocardial infarction (MI) and cardiac arrest based on increasing age, American Society of Anesthesiologists class, type of surgery, dependent functional status, and abnormal serum creatinine level. However, given its accuracy and simplicity, the revised cardiac risk index (RCRI) (Table 9-3) is favored. The RCRI relies on the presence or absence of six identifiable predictive factors: high-risk surgery, ischemic heart disease, congestive heart failure, cerebrovascular disease, diabetes mellitus, and renal dysfunction. Each of these predictors is assigned one point. The risk of major cardiac events—defined as myocardial infarction, pulmonary edema, ventricular fibrillation or primary cardiac arrest, and complete heart block—can then be predicted. Based on the presence of none, one, two, three, or more of these clinical predictors, the rate of development of one of these four major cardiac events is estimated to be 0.4, 0.9, 7, and 11%, respectively (Fig. 9-2). An RCRI score of 0 signifies a 0.4–0.5% risk of cardiac events; RCRI 1, 0.9–1.3%; RCRI 2, 4–7%; and RCRI ≥3, 9–11%. The clinical utility of the RCRI is to identify patients with three or more predictors who are at very high risk (≥11%) for cardiac complications and who may benefit from further risk stratification with noninvasive cardiac testing or initiation of preoperative preventive medical management.
CLINICAL MARKERS INCLUDED IN THE REVISED CARDIAC RISK INDEX |
Abbreviations: CABG, coronary artery bypass grafting; ECG, electrocardiogram; PCI, percutaneous coronary interventions.
Source: Adapted from TH Lee et al: Circulation 100:1043, 1999.
FIGURE 9-2 Risk stratification based on the RCRI: derivation and prospective validation of a simple index for prediction of cardiac risk in patients undergoing major noncardiac surgery. Cardiac events include myocardial infarction, pulmonary edema, ventricular fibrillation, cardiac asystole, and complete heart block. (Adapted from TH Lee et al: Circulation 100:1043, 1999.)
PREOPERATIVE NONINVASIVE CARDIAC TESTING FOR RISK STRATIFICATION
There is little evidence to support widespread application of preoperative noninvasive cardiac testing for all patients undergoing major surgery. Rather, a discriminative approach based on clinical risk categorization appears to be both clinically useful and cost-effective. There is potential benefit in identifying asymptomatic but high-risk patients, such as those with left main or left main–equivalent CAD or those with three-vessel CAD and poor left ventricular function, who may benefit from coronary revascularization (Chap. 293). However, evidence does not support aggressive attempts to identify patients at intermediate risk who have asymptomatic but advanced coronary artery disease, in whom coronary revascularization appears to offer little advantage over medical therapy.
An RCRI score ≥3 in patients with severe myocardial ischemia on stress testing should lead to consideration of coronary revascularization prior to noncardiac surgery. Noninvasive cardiac testing is most appropriate if it is anticipated that, in the event of a strongly positive test, a patient will meet guidelines for coronary angiography and coronary revascularization. Pharmacologic stress tests are more useful than exercise testing in patients with functional limitations. Dobutamine echocardiography and persantine, adenosine, or dobutamine nuclear perfusion testing (Chap. 270e) have excellent negative predictive values (near 100%) but poor positive predictive values (<20%) in the identification of patients at risk for perioperative MI or death. Thus, a negative study is reassuring, but a positive study is a relatively weak predictor of a “hard” perioperative cardiac event.
RISK MODIFICATION: PREVENTIVE STRATEGIES TO REDUCE CARDIAC RISK
Perioperative Coronary Revascularization Currently, potential options for reducing perioperative cardiovascular risk include coronary artery revascularization and/or perioperative preventive medical therapies (Chap. 293). Prophylactic coronary revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) provides no short- or midterm survival benefit for patients without left main CAD or three-vessel CAD in the presence of poor left ventricular systolic function and is not recommended for patients with stable CAD before noncardiac surgery. Although PCI is associated with lower procedural risk than is CABG in the perioperative setting, the placement of a coronary artery stent soon before noncardiac surgery may increase the risk of bleeding during surgery if dual antiplatelet therapy (aspirin and thienopyridine) is administered; moreover, stent placement shortly before noncardiac surgery increases the perioperative risk of MI and cardiac death due to stent thrombosis if such therapy is withdrawn prematurely (Chap. 296e). It is recommended that, if possible, noncardiac surgery be delayed 30–45 days after placement of a bare metal coronary stent and for 365 days after a drug-eluting stent. For patients who must undergo noncardiac surgery early (>14 days) after PCI, balloon angioplasty without stent placement appears to be a reasonable alternative because dual antiplatelet therapy is not necessary in such patients. One recent clinical trial further suggests that after 6 months, bare metal and drug eluting stents may not pose a threat.
PERIOPERATIVE PREVENTIVE MEDICAL THERAPIES The goal of perioperative preventive medical therapies with β-adrenergic antagonists, HMG-CoA reductase inhibitors (statins), antiplatelet agents, and α2 agonists is to reduce perioperative adrenergic stimulation, ischemia, and inflammation, which are triggered during the perioperative period.
β-ADRENERGIC ANTAGONISTS The use of perioperative beta blockade should be based on a thorough assessment of a patient’s perioperative clinical and surgery-specific cardiac risk (RCRI ≥2). For patients with or without mild to moderate reactive airway disease, the cardioselective beta blocker of choice should be used and titrated to maintain a target heart rate of 60–80 beats/min in the absence of hypotension in the operative and perioperative period. In RCRI ≥2 patients without a long-term indication for beta blockers, the medications can be administered IV as a preoperative medication on the day of surgery, with a targeted heart rate of 60–80 beats/min without hypotension, and continued for >7 days (preferably 30 days) postoperatively. Abrupt perioperative beta blocker withdrawal should be avoided unless necessary because of the associated increase in risk of MI and angina. IV preparations should be substituted for oral medications if patients are unable to swallow or absorb pills in the perioperative period. The results from the Perioperative Ischemic Evaluation (POISE) trial showed that, although cardiac death, nonfatal myocardial infarction, or cardiac arrest was reduced among patients who received metoprolol rather than placebo, there was an increased incidence of death and stroke among metoprolol recipients because of a high and rapidly loading dose of this drug. The POISE trial highlights the importance of a clear risk-and-benefit assessment, with careful initiation and titration to therapeutic efficacy of preoperative beta blockers in patients undergoing noncardiac surgery. A recent meta-analysis which included the POISE study further supports that excessive beta blocker dosing is, in fact, harmful.
The ACC/AHA guidelines recommend the following: (1) Beta blockers should be continued in patients with active cardiac conditions who are undergoing surgery and are receiving beta blockers. (2) Beta blockers titrated to heart rate and blood pressure are probably recommended for patients undergoing vascular surgery who are at high cardiac risk defined by CAD or cardiac ischemia on preoperative testing. (3) Beta blockers are reasonable for high-risk patients (RCRI ≥2) who undergo vascular surgery. (4) Beta blockers are reasonable for patients with known CAD or high risk (RCRI ≥2) who undergo intermediate-risk surgery. (5) Nondiscriminant administration of high-dose beta blockers without dose titration to effectiveness is contraindicated for patients who have never been treated with a beta blocker.
HMG-CoA REDUCTASE INHIBITORS (STATINS) A number of prospective and retrospective studies support the perioperative prophylactic use of statins for reduction of cardiac complications in patients with established atherosclerosis. The ACC/AHA Guidelines support the protective efficacy of perioperative statins on cardiac complications in intermediate risk patients undergoing major noncardiac surgery. For patients undergoing noncardiac surgery and currently taking statins, statin therapy should be continued to reduce perioperative cardiac risk. Statins are reasonable for patients undergoing vascular surgery with or without clinical risk factors (RCRI ≥1).
ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS Evidence supports the discontinuation of ACE inhibitors and angiotensin receptor blockers for 24 h prior to noncardiac surgery due to adverse circulatory effects after induction of anesthesia.
ORAL ANTIPLATELET AGENTS Evidence-based recommendations regarding perioperative use of aspirin and/or thienopyridine to reduce cardiac risk currently lack clarity. A substantial increase in perioperative bleeding and in the need for transfusion in patients receiving dual antiplatelet therapy has been observed. The discontinuation of thienopyridine and aspirin for 5–7 days prior to major surgery to minimize the risk of perioperative bleeding and transfusion must be balanced with the potential increased risk of an acute coronary syndrome and of subacute stent thrombosis in patients with recent coronary stent implantation. If clinicians elect to withhold antiplatelet agents prior to surgery, these agents should be restarted as soon as possible postoperatively.
α2 AGONISTS Several prospective and retrospective meta-analyses of perioperative α2 agonists (clonidine and mivazerol) demonstrated a reduction of cardiac death rates among patients with known coronary artery disease who underwent noncardiac surgery. α2 agonists thus may be considered for perioperative control of hypertension in patients with known coronary artery disease or an RCRI score ≥2.
CALCIUM CHANNEL BLOCKERS Evidence is lacking to support the use of calcium channel blockers as a prophylactic strategy to decrease perioperative risk in major noncardiac surgery.
ANESTHETICS Mortality risk is low with safe delivery of modern anesthesia, especially among low-risk patients undergoing low-risk surgery (Table 9-4). Inhaled anesthetics have predictable circulatory and respiratory effects: all decrease arterial pressure in a dose-dependent manner by reducing sympathetic tone and causing systemic vasodilation, myocardial depression, and decreased cardiac output. Inhaled anesthetics also cause respiratory depression, with diminished responses to both hypercapnia and hypoxemia, in a dose-dependent manner; in addition, these agents have a variable effect on heart rate. Prolonged residual neuromuscular blockade also increases the risk of postoperative pulmonary complications due to reduction in functional residual lung capacity, loss of diaphragmatic and intercostal muscle function, atelectasis, and arterial hypoxemia from ventilation-perfusion mismatch.
GRADATION OF MORTALITY RISK OF COMMON NONCARDIAC SURGICAL PROCEDURES |

Several meta-analyses have shown that rates of pneumonia and respiratory failure are lower among patients receiving neuroaxial anesthesia (epidural or spinal) rather than general anesthesia (inhaled). However, there were no significant differences in cardiac events between the two approaches. Evidence from a meta-analysis of randomized controlled trials supports postoperative epidural analgesia for >24 h for the purpose of pain relief. However, the risk of epidural hematoma in the setting of systemic anticoagulation for venous thromboembolism prophylaxis (see below) and postoperative epidural catheterization must be considered.
PREOPERATIVE PULMONARY RISK ASSESSMENT
Perioperative pulmonary complications occur frequently and lead to significant morbidity and mortality. The guidelines from the American College of Physicians recommend the following:
1. All patients undergoing noncardiac surgery should be assessed for risk of pulmonary complications (Table 9-5).
PREDISPOSING RISK FACTORS FOR PULMONARY COMPLICATIONS |
e. PO2 ≤50 mmHg
Abbreviations: FEV1, forced expiratory volume in 1 s; MVV, maximal voluntary ventilation; PEF, peak expiratory flow rate; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen.
Source: A Qaseem et al: Ann Intern Med 144:575-80. Modified from GW Smetana et al: Ann Intern Med 144:581, 2006, and from DN Mohr et al: Postgrad Med 100:247, 1996.
2. Patients undergoing emergency or prolonged (3- to 4-h) surgery; aortic aneurysm repair; vascular surgery; major abdominal, thoracic, neurologic, head, or neck surgery; and general anesthesia should be considered to be at elevated risk for postoperative pulmonary complications.
3. Patients at higher risk of pulmonary complications should undergo incentive spirometry, deep-breathing exercises, cough encouragement, postural drainage, percussion and vibration, suctioning and ambulation, intermittent positive-pressure breathing, continuous positive airway pressure, and selective use of a nasogastric tube for postoperative nausea, vomiting, or symptomatic abdominal distention to reduce postoperative risk (Table 9-6).
RISK MODIFICATION TO REDUCE PERIOPERATIVE PULMONARY COMPLICATIONS |
Source: From VA Lawrence et al: Ann Intern Med 144:596, 2006, and WF Dunn, PD Scanlon: Mayo Clin Proc 68:371, 1993.
4. Routine preoperative spirometry and chest radiography should not be used routinely for predicting risk of postoperative pulmonary complications but may be appropriate for patients with chronic obstructive pulmonary disease or asthma.
5. Spirometry is of value before lung resection in determining candidacy for coronary artery bypass; however, it does not provide a spirometric threshold for extrathoracic surgery below which the risks of surgery are unacceptable.
6. Pulmonary artery catheterization, administration of total parenteral nutrition (as opposed to no supplementation), or total enteral nutrition has no benefit in reducing postoperative pulmonary complications.
PERIOPERATIVE MANAGEMENT AND PROPHYLAXIS
DIABETES MELLITUS
(See also Chaps. 417–419) Many patients with diabetes mellitus have significant symptomatic or asymptomatic CAD and may have silent myocardial ischemia due to autonomic dysfunction. Evidence supports intensive perioperative glycemic control to achieve near-normal glucose levels (90–110 mg/dL) rather than moderate glycemic control (120–200 mg/dL), using insulin infusion. This practice must be balanced against the risk of hypoglycemic complications. Oral hypoglycemic agonists should not be given on the morning of surgery. Perioperative hyperglycemia should be treated with IV infusion of short-acting insulin or SC sliding-scale insulin. Patients whose diabetes is diet controlled may proceed to surgery with close postoperative monitoring.
INFECTIVE ENDOCARDITIS
(See also Chap. 155) Perioperative prophylactic antibiotics should be administered to patients with congenital or valvular heart disease, prosthetic valves, mitral valve prolapse, or other cardiac abnormalities, in accordance with ACC/AHA practice guidelines.
VENOUS THROMBOEMBOLISM
(See also Chap. 300) Perioperative prophylaxis of venous thromboembolism should follow established guidelines of the American College of Chest Physicians. Aspirin is not supported as a single agent for thromboprophylaxis. Low-dose unfractionated heparin (≤5000 units SC bid), low-molecular weight heparin (e.g., enoxaparin, 30 mg bid or 40 mg qd), or a pentasaccharide (fondaparinux, 2.5 mg qd) is appropriate for patients at moderate risk; unfractionated heparin (5000 units SC tid) is appropriate for patients at high risk. Graduated compression stockings and pneumatic compression devices are useful supplements to anticoagulant therapy.
10 | Palliative and End-of-Life Care |
EPIDEMIOLOGY
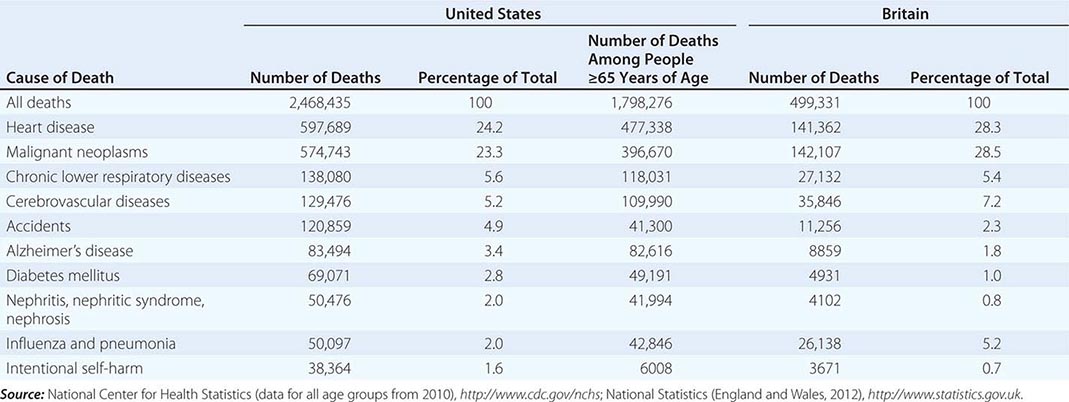
In 2010, according to the Centers for Disease Control and Prevention, 2,468,435 individuals died in the United States (Table 10-1). Approximately 73% of all deaths occur in those >65 years of age. The epidemiology of mortality is similar in most developed countries; cardiovascular diseases and cancer are the predominant causes of death, a marked change since 1900, when heart disease caused ~8% of all deaths and cancer accounted for <4% of all deaths. In 2010, the year with the most recent available data, AIDS did not rank among the top 15 causes of death, causing just 8369 deaths. Even among people age 35–44, heart disease, cancer, chronic liver disease, and accidents all cause more deaths than AIDS.
TEN LEADING CAUSES OF DEATH IN THE UNITED STATES AND BRITAIN |
It is estimated that in developed countries ~70% of all deaths are preceded by a disease or condition, making it reasonable to plan for dying in the foreseeable future. Cancer has served as the paradigm for terminal care, but it is not the only type of illness with a recognizable and predictable terminal phase. Because heart failure, chronic obstructive pulmonary disease (COPD), chronic liver failure, dementia, and many other conditions have recognizable terminal phases, a systematic approach to end-of-life care should be part of all medical specialties. Many patients with illness-related suffering also can benefit from palliative care regardless of prognosis. Ideally, palliative care should be considered part of comprehensive care for all patients. Palliative care can be improved by coordination between caregivers, doctors, and patients for advance care planning, as well as dedicated teams of physicians, nurses, and other providers.
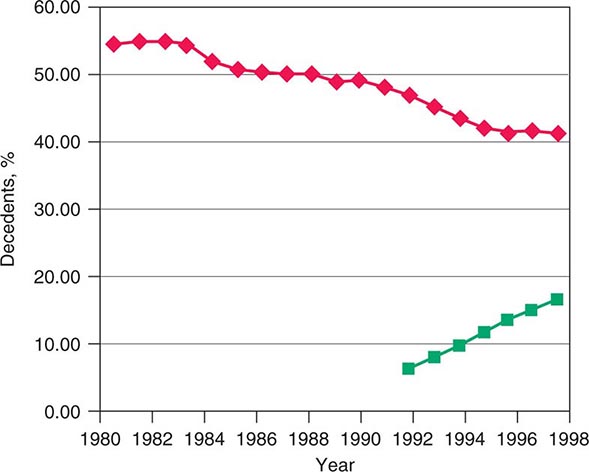
The rapid increases in life expectancy in developed countries over the last century have been accompanied by new difficulties facing individuals, families, and society as a whole in addressing the needs of an aging population. These challenges include both more complicated conditions and technologies to address them at the end of life. The development of technologies that can prolong life without restoring full health has led many Americans to seek out alternative end-of-life care settings and approaches that relieve suffering for those with terminal diseases. Over the last few decades in the United States, a significant change in the site of death has occurred that coincides with patient and family preferences. Nearly 60% of Americans died as inpatients in hospitals in 1980. By 2000, the trend was reversing, with ~31% of Americans dying as hospital inpatients (Fig. 10-1). This shift has been most dramatic for those dying from cancer and COPD and for younger and very old individuals. In the last decade, it has been associated with the increased use of hospice care; in 2008, approximately 39% of all decedents in the United States received such care. Cancer patients currently constitute ~36.9% of hospice users. About 79% of patients receiving hospice care die out of the hospital, and around 42% of those receiving hospice care die in a private residence. In addition, in 2008, for the first time, the American Board of Medical Specialties (ABMS) offered certification in hospice and palliative medicine. With shortening of hospital stays, many serious conditions are being treated at home or on an outpatient basis. Consequently, providing optimal palliative and end-of-life care requires ensuring that appropriate services are available in a variety of settings, including noninstitutional settings.
FIGURE 10-1 Graph showing trends in the site of death in the last two decades. ![]() , percentage of hospital inpatient deaths;
, percentage of hospital inpatient deaths; ![]() , percentage of decedents enrolled in a hospice.
, percentage of decedents enrolled in a hospice.
HOSPICE AND THE PALLIATIVE CARE FRAMEWORK
Central to this type of care is an interdisciplinary team approach that typically encompasses pain and symptom management, spiritual and psychological care for the patient, and support for family caregivers during the patient’s illness and the bereavement period.
Terminally ill patients have a wide variety of advanced diseases, often with multiple symptoms that demand relief, and require noninvasive therapeutic regimens to be delivered in flexible care settings. Fundamental to ensuring quality palliative and end-of-life care is a focus on four broad domains: (1) physical symptoms; (2) psychological symptoms; (3) social needs that include interpersonal relationships, caregiving, and economic concerns; and (4) existential or spiritual needs.
A comprehensive assessment screens for and evaluates needs in each of these four domains. Goals for care are established in discussions with the patient and/or family, based on the assessment in each of the domains. Interventions then are aimed at improving or managing symptoms and needs. Although physicians are responsible for certain interventions, especially technical ones, and for coordinating the interventions, they cannot be responsible for providing all of them. Because failing to address any one of the domains is likely to preclude a good death, a well-coordinated, effectively communicating interdisciplinary team takes on special importance in end-of-life care. Depending on the setting, critical members of the interdisciplinary team will include physicians, nurses, social workers, chaplains, nurse’s aides, physical therapists, bereavement counselors, and volunteers.
ASSESSMENT AND CARE PLANNING
Comprehensive Assessment Standardized methods for conducting a comprehensive assessment focus on evaluating the patient’s condition in all four domains affected by illness: physical, psychological, social, and spiritual. The assessment of physical and mental symptoms should follow a modified version of the traditional medical history and physical examination that emphasizes symptoms. Questions should aim at elucidating symptoms and discerning sources of suffering and gauging how much those symptoms interfere with the patient’s quality of life. Standardized assessment is critical. Currently, there are 21 symptom assessment instruments for cancer alone. Further research on and validation of these assessment tools, especially taking into account patient perspectives, could improve their effectiveness. Instruments with good psychometric properties that assess a wide range of symptoms include the Memorial Symptom Assessment Scale (MSAS), the Rotterdam Symptom Checklist, the Worthing Chemotherapy Questionnaire, and the Computerized Symptom Assessment Instrument. These instruments are long and may be useful for initial clinical or for research assessments. Shorter instruments are useful for patients whose performance status does not permit comprehensive assessments. Suitable shorter instruments include the Condensed Memorial Symptom Assessment Scale, the Edmonton Symptom Assessment System, the M.D. Anderson Symptom Assessment Inventory, and the Symptom Distress Scale. Using such instruments ensures that the assessment is comprehensive and does not focus only on pain and a few other physical symptoms. Invasive tests are best avoided in end-of-life care, and even minimally invasive tests should be evaluated carefully for their benefit-to-burden ratio for the patient. Aspects of the physical examination that are uncomfortable and unlikely to yield useful information can be omitted.
Regarding social needs, health care providers should assess the status of important relationships, financial burdens, caregiving needs, and access to medical care. Relevant questions will include the following: How often is there someone to feel close to? How has this illness been for your family? How has it affected your relationships? How much help do you need with things like getting meals and getting around? How much trouble do you have getting the medical care you need? In the area of existential needs, providers should assess distress and the patient’s sense of being emotionally and existentially settled and of finding purpose or meaning. Helpful assessment questions can include the following: How much are you able to find meaning since your illness began? What things are most important to you at this stage? In addition, it can be helpful to ask how the patient perceives his or her care: How much do you feel your doctors and nurses respect you? How clear is the information from us about what to expect regarding your illness? How much do you feel that the medical care you are getting fits with your goals? If concern is detected in any of these areas, deeper evaluative questions are warranted.
Communication Especially when an illness is life-threatening, there are many emotionally charged and potentially conflict-creating moments, collectively called “bad news” situations, in which empathic and effective communication skills are essential. Those moments include communicating with the patient and/or family about a terminal diagnosis, the patient’s prognosis, any treatment failures, deemphasizing efforts to cure and prolong life while focusing more on symptom management and palliation, advance care planning, and the patient’s death. Although these conversations can be difficult and lead to tension, research indicates that end-of-life discussions can lead to earlier hospice referrals rather than overly aggressive treatment, benefiting quality of life for patients and improving the bereavement process for families.
Just as surgeons plan and prepare for major operations and investigators rehearse a presentation of research results, physicians and health care providers caring for patients with significant or advanced illness can develop a practiced approach to sharing important information and planning interventions. In addition, families identify as important both how well the physician was prepared to deliver bad news and the setting in which it was delivered. For instance, 27% of families making critical decisions for patients in an intensive care unit (ICU) desired better and more private physical space to communicate with physicians, and 48% found having clergy present reassuring.
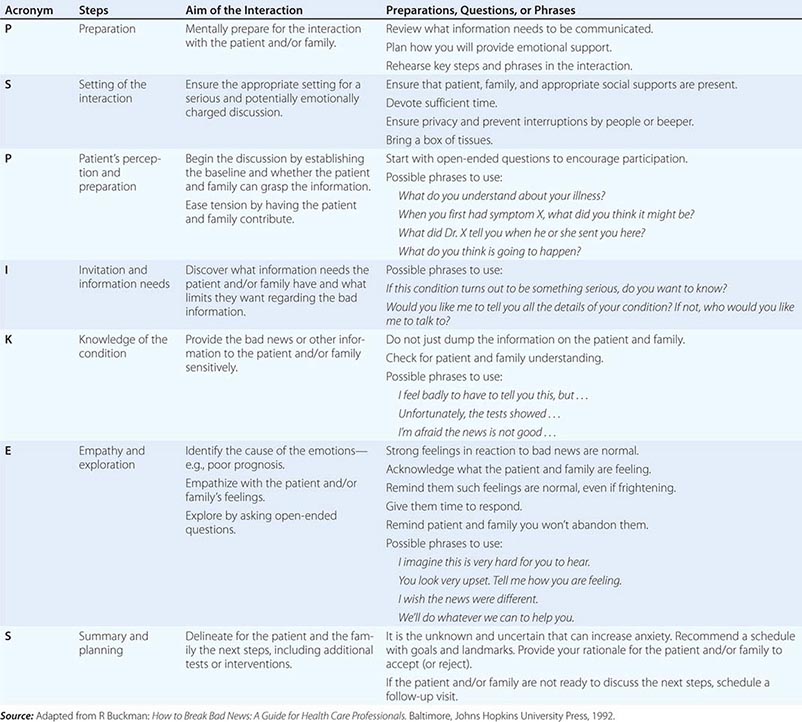
An organized and effective seven-step procedure for communicating bad news goes by the acronym P-SPIKES: (1) prepare for the discussion, (2) set up a suitable environment, (3) begin the discussion by finding out what the patient and/or family understand, (4) determine how they will comprehend new information best and how much they want to know, (5) provide needed new knowledge accordingly, (6) allow for emotional responses, and (7) share plans for the next steps in care. Table 10-2 provides a summary of these steps along with suggested phrases and underlying rationales for each one. Additional research that further considers the response of patients to systematic methods of delivering bad news could build the evidence base for even more effective communication procedures.
ELEMENTS OF COMMUNICATING BAD NEWS—THE P-SPIKES APPROACH |
Continuous Goal Assessment Major barriers to ensuring quality palliative and end-of-life care include difficulty providing an accurate prognosis and emotional resistance of patients and their families to accepting the implications of a poor prognosis. There are two practical solutions to these barriers. One is to integrate palliative care with curative care regardless of prognosis. With this approach, palliative care no longer conveys the message of failure, having no more treatments, or “giving up hope.” Fundamental to integrating palliative care with curative therapy is to include continuous goal assessment as part of the routine patient reassessment that occurs at most patient-physician encounters. Alternatively, some practices may find it useful to implement a standard point in the clinical course to address goals of care and advance care planning. For example, some oncology practices ask all patients whose Eastern Cooperative Oncology Group (ECOG) performance status is 3 or less—meaning they spend 50% or more of the day in bed—or those who develop metastatic disease about their goals of care and advance care preferences.
Goals for care are numerous, ranging from cure of a specific disease, to prolonging life, to relief of a symptom, to delaying the course of an incurable disease, to adapting to progressive disability without disrupting the family, to finding peace of mind or personal meaning, to dying in a manner that leaves loved ones with positive memories. Discernment of goals for care can be approached through a seven-step protocol: (1) ensure that medical and other information is as complete as reasonably possible and is understood by all relevant parties (see above); (2) explore what the patient and/or family are hoping for while identifying relevant and realistic goals; (3) share all the options with the patient and family; (4) respond with empathy as they adjust to changing expectations; (5) make a plan, emphasizing what can be done toward achieving the realistic goals; (6) follow through with the plan; and (7) review and revise the plan periodically, considering at every encounter whether the goals of care should be reviewed with the patient and/or family. Each of these steps need not be followed in rote order, but together they provide a helpful framework for interactions with patients and their families about goals for care. It can be especially challenging if a patient or family member has difficulty letting go of an unrealistic goal. One strategy is to help them refocus on more realistic goals and also suggest that while hoping for the best, it is still prudent to plan for other outcomes as well.
Advance Care Planning • PRACTICES Advance care planning is a process of planning for future medical care in case the patient becomes incapable of making medical decisions. A 2010 study of adults 60 or older who died between 2000 and 2006 found that 42% required decision making about treatment in the final days of life but 70% lacked decision-making capacity. Among those lacking decision-making capacity, around one-third did not have advance planning directives. Ideally, such planning would occur before a health care crisis or the terminal phase of an illness. Diverse barriers prevent this. Polls suggest 80% of Americans endorse advance care planning and completing living wills. However, data suggest between 33 and 42% have actually completed one. Other countries have even lower completion rates. Most patients expect physicians to initiate advance care planning and will wait for physicians to broach the subject. Patients also wish to discuss advance care planning with their families. Yet patients with unrealistic expectations are significantly more likely to prefer aggressive treatments. Fewer than one-third of health care providers have completed advance care planning for themselves. Hence, a good first step is for health care providers to complete their own advance care planning. This makes providers aware of the critical choices in the process and the issues that are especially charged and allows them to tell their patients truthfully that they personally have done advance planning. Lessons from behavioral economics suggest that setting this kind of social norming helps people view completing an advance directive as acceptable and even expected.
Steps in effective advance care planning center on (1) introducing the topic, (2) structuring a discussion, (3) reviewing plans that have been discussed by the patient and family, (4) documenting the plans, (5) updating them periodically, and (6) implementing the advance care directives (Table 10-3). Two of the main barriers to advance care planning are problems in raising the topic and difficulty in structuring a succinct discussion. Raising the topic can be done efficiently as a routine matter, noting that it is recommended for all patients, analogous to purchasing insurance or estate planning. Many of the most difficult cases have involved unexpected, acute episodes of brain damage in young individuals.
STEPS IN ADVANCE CARE PLANNING |
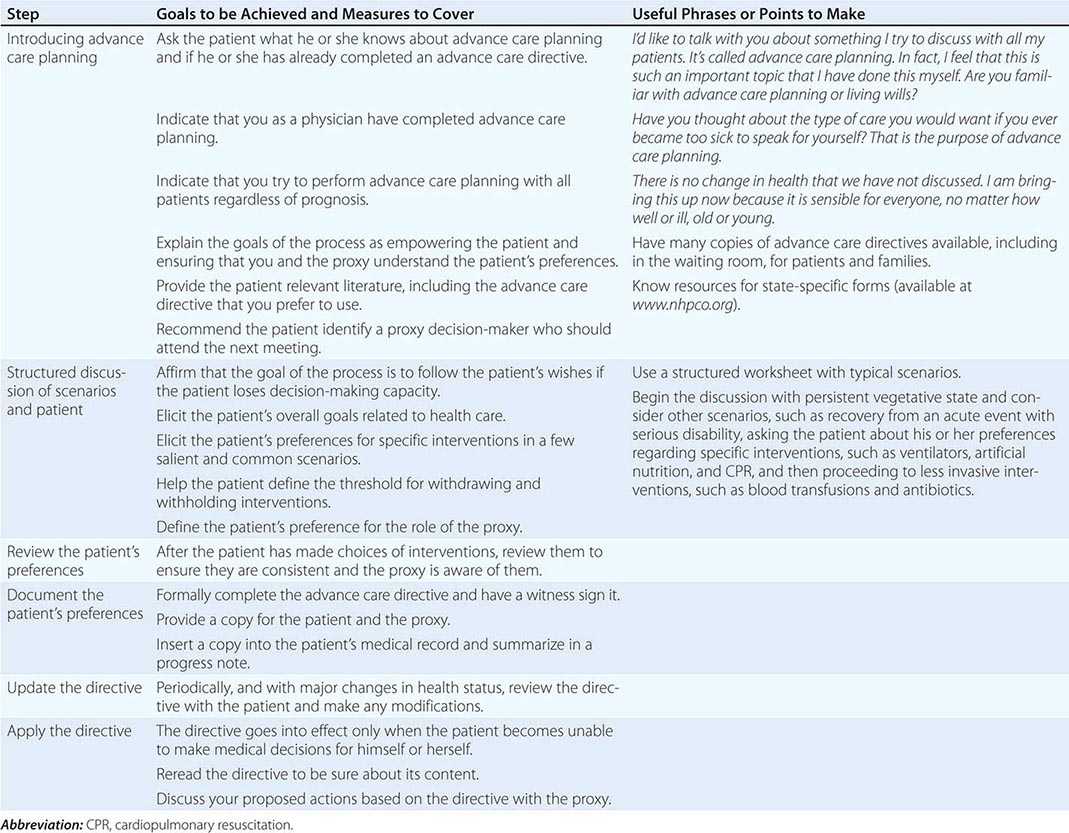
Structuring a focused discussion is a central communication skill. Identify the health care proxy and recommend his or her involvement in the process of advance care planning. Select a worksheet, preferably one that has been evaluated and demonstrated to produce reliable and valid expressions of patient preferences, and orient the patient and proxy to it. Such worksheets exist for both general and disease-specific situations. Discuss with the patient and proxy one scenario as an example to demonstrate how to think about the issues. It is often helpful to begin with a scenario in which the patient is likely to have settled preferences for care, such as being in a persistent vegetative state. Once the patient’s preferences for interventions in this scenario are determined, suggest that the patient and proxy discuss and complete the worksheet for the others. If appropriate, suggest that they involve other family members in the discussion. On a return visit, go over the patient’s preferences, checking and resolving any inconsistencies. After having the patient and proxy sign the document, place it in the medical chart and be sure that copies are provided to relevant family members and care sites. Because patients’ preferences can change, these documents have to be reviewed periodically.
TYPES OF DOCUMENTS Advance care planning documents are of three broad types. The first includes living wills or instructional directives; these are advisory documents that describe the types of decisions that should direct care. Some are more specific, delineating different scenarios and interventions for the patient to choose from. Among these, some are for general use and others are designed for use by patients with a specific type of disease, such as cancer or HIV. A second type is a less specific directive that provides general statements of not wanting life-sustaining interventions or forms that describe the values that should guide specific discussions about terminal care. These can be problematic because, when critical decisions about specific treatments are needed, they require assessments by people other than the patient of whether a treatment fulfills a particular wish. The third type of advance directive allows the designation of a health care proxy (sometimes also referred to as a durable attorney for health care), who is an individual selected by the patient to make decisions. The choice is not either/or; a combined directive that includes a living will and designates a proxy is often used, and the directive should indicate clearly whether the specified patient preferences or the proxy’s choice takes precedence if they conflict. The Five Wishes and the Medical Directive are such combined forms. Some states have begun to put into practice a “Physician Orders for Life-Sustaining Treatment (POLST)” paradigm, which builds on communication between providers and patients to include guidance for end-of-life care in a color-coordinated form that follows the patient across treatment settings. The procedures for completing advance care planning documents vary according to state law.
A potentially misleading distinction relates to statutory as opposed to advisory documents. Statutory documents are drafted to fulfill relevant state laws. Advisory documents are drafted to reflect the patient’s wishes. Both are legal, the first under state law and the latter under common or constitutional law.
LEGAL ASPECTS The U.S. Supreme Court has ruled that patients have a constitutional right to decide about refusing and terminating medical interventions, including life-sustaining interventions, and that mentally incompetent patients can exercise this right by providing “clear and convincing evidence” of their preferences. Because advance care directives permit patients to provide such evidence, commentators agree that they are constitutionally protected. Most commentators believe that a state is required to honor any clear advance care directive whether or not it is written on an “official” form. Many states have enacted laws explicitly to honor out-of-state directives. If a patient is not using a statutory form, it may be advisable to attach a statutory form to the advance care directive being used. State-specific forms are readily available free of charge for health care providers and patients and families through the National Hospice and Palliative Care Organization’s “Caring Connections” website (http://www.caringinfo.org).
In January 2014, Texas judge R. H. Wallace ruled that a brain dead woman who was 23 weeks pregnant should be removed from life support. This was after several months of disagreement between the woman’s family and the hospital providing care. The hospital cited Texas law that states that life-sustaining treatment must be administered to a pregnant woman, but the judge sided with the woman’s family saying that the law did not apply because the patient was legally dead.
As of 2013, advance directives are legal in all states and the District of Columbia either through state specific legislation, state judicial rulings, or United States Supreme Court rulings. Many states have their own statutory forms. Massachusetts and Michigan do not have living will laws, although both have health care proxy laws. In 27 states, the laws state that the living will is not valid if a woman is pregnant. However, like all other states except Alaska, these states have enacted durable power of attorney for health care laws that permit patients to designate a proxy decision-maker with authority to terminate life-sustaining treatments. Only in Alaska does the law prohibit proxies from terminating life-sustaining treatments. The health reform legislation, the Affordable Care Act of 2010, raised substantial controversy when early versions of the law included Medicare reimbursement for advance care planning consultations. These provisions were withdrawn over accusations that they would lead to the rationing of care for the elderly.
INTERVENTIONS
PHYSICAL SYMPTOMS AND THEIR MANAGEMENT
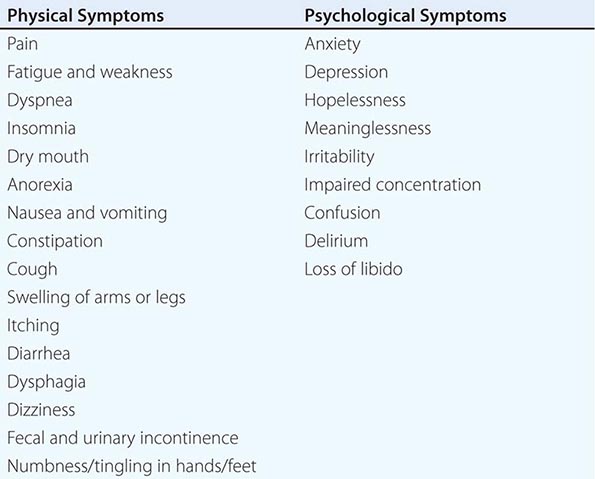
Great emphasis has been placed on addressing dying patients’ pain. Some institutions have made pain assessment a fifth vital sign to emphasize its importance. This also has been advocated by large health care systems such as the Veterans’ Administration and accrediting bodies such as the Joint Commission. Although this embrace of pain as the fifth vital sign has been symbolically important, no data document that it has improved pain management practices. Although good palliative care requires good pain management, it also requires more. The frequency of symptoms varies by disease and other factors. The most common physical and psychological symptoms among all terminally ill patients include pain, fatigue, insomnia, anorexia, dyspnea, depression, anxiety, and nausea and vomiting. In the last days of life, terminal delirium is also common. Assessments of patients with advanced cancer have shown that patients experienced an average of 11.5 different physical and psychological symptoms (Table 10-4).
COMMON PHYSICAL AND PSYCHOLOGICAL SYMPTOMS OF TERMINALLY ILL PATIENTS |
Evaluations to determine the etiology of these symptoms usually can be limited to the history and physical examination. In some cases, radiologic or other diagnostic examinations will provide sufficient benefit in directing optimal palliative care to warrant the risks, potential discomfort, and inconvenience, especially to a seriously ill patient. Only a few of the common symptoms that present difficult management issues will be addressed in this chapter. Additional information on the management of other symptoms, such as nausea and vomiting, insomnia, and diarrhea, can be found in Chaps. 54 and 99, Chap. 38, and Chap. 55, respectively.
Pain • FREQUENCY The frequency of pain among terminally ill patients varies widely. Substantial pain occurs in 36–90% of patients with advanced cancer. In the SUPPORT study of hospitalized patients with diverse conditions and an estimated survival ≤6 months, 22% reported moderate to severe pain, and caregivers of those patients noted that 50% had similar levels of pain during the last few days of life. A meta-analysis found pain prevalence of 58–69% in studies that included patients characterized as having advanced, metastatic, or terminal cancer; 44–73% in studies that included patients characterized as undergoing cancer treatment; and 21–46% in studies that included posttreatment individuals.
ETIOLOGY Nociceptive pain is the result of direct mechanical or chemical stimulation of nociceptors and normal neural signaling to the brain. It tends to be localized, aching, throbbing, and cramping. The classic example is bone metastases. Visceral pain is caused by nociceptors in gastrointestinal, respiratory, and other organ systems. It is a deep or colicky type of pain classically associated with pancreatitis, myocardial infarction, or tumor invasion of viscera. Neuropathic pain arises from disordered nerve signals. It is described by patients as burning, electrical, or shocklike pain. Classic examples are poststroke pain, tumor invasion of the brachial plexus, and herpetic neuralgia.
ASSESSMENT Pain is a subjective experience. Depending on the patient’s circumstances, perspective, and physiologic condition, the same physical lesion or disease state can produce different levels of reported pain and need for pain relief. Systematic assessment includes eliciting the following: (1) type: throbbing, cramping, burning, etc.; (2) periodicity: continuous, with or without exacerbations, or incident; (3) location; (4) intensity; (5) modifying factors; (6) effects of treatments; (7) functional impact; and (8) impact on patient. Several validated pain assessment measures may be used, such as the Visual Analogue Scale, the Brief Pain Inventory, and the pain component of one of the more comprehensive symptom assessment instruments. Frequent reassessments are essential to assess the effects of interventions.
INTERVENTIONS Interventions for pain must be tailored to each individual, with the goal of preempting chronic pain and relieving breakthrough pain. At the end of life, there is rarely reason to doubt a patient’s report of pain. Pain medications are the cornerstone of management. If they are failing and nonpharmacologic interventions—including radiotherapy and anesthetic or neurosurgical procedures such as peripheral nerve blocks or epidural medications—are required, a pain consultation is appropriate.
Pharmacologic interventions follow the World Health Organization three-step approach involving nonopioid analgesics, mild opioids, and strong opioids, with or without adjuvants (Chap. 18). Nonopioid analgesics, especially nonsteroidal anti-inflammatory drugs (NSAIDs), are the initial treatments for mild pain. They work primarily by inhibiting peripheral prostaglandins and reducing inflammation but also may have central nervous system (CNS) effects. They have a ceiling effect. Ibuprofen, up to a total dose of 1600 mg/d given in four doses of 400 mg each, has a minimal risk of causing bleeding and renal impairment and is a good initial choice. In patients with a history of severe gastrointestinal (GI) or other bleeding, it should be avoided. In patients with a history of mild gastritis or gastroesophageal reflux disease (GERD), acid-lowering therapy such as a proton pump inhibitor should be used. Acetaminophen is an alternative in patients with a history of GI bleeding and can be used safely at up to 4 g/d given in four doses of 1 g each. In patients with liver dysfunction due to metastases or other causes and in patients with heavy alcohol use, doses should be reduced.
If nonopioid analgesics are insufficient, opioids should be introduced. They work by interacting with µ opioid receptors in the CNS to activate pain-inhibitory neurons; most are receptor antagonists. The mixed agonist/antagonist opioids useful for postacute pain should not be used for the chronic pain in end-of-life care. Weak opioids such as codeine can be used initially. However, if they are escalated and fail to relieve pain, strong opioids such as morphine, 5–10 mg every 4 h, should be used. Nonopioid analgesics should be combined with opioids because they potentiate the effect of opioids.
For continuous pain, opioids should be administered on a regular, around-the-clock basis consistent with their duration of analgesia. They should not be provided only when the patient experiences pain; the goal is to prevent patients from experiencing pain. Patients also should be provided rescue medication, such as liquid morphine, for breakthrough pain, generally at 20% of the baseline dose. Patients should be informed that using the rescue medication does not obviate the need to take the next standard dose of pain medication. If after 24 h the patient’s pain remains uncontrolled and recurs before the next dose, requiring the patient to use the rescue medication, the daily opioid dose can be increased by the total dose of rescue medications used by the patient, or by 50% for moderate pain and 100% for severe pain of the standing opioid daily dose.
It is inappropriate to start with extended-release preparations. Instead, an initial focus on using short-acting preparations to determine how much is required in the first 24–48 h will allow clinicians to determine opioid needs. Once pain relief is obtained with short-acting preparations, one should switch to extended-release preparations. Even with a stable extended-release preparation regimen, the patient may have incident pain, such as during movement or dressing changes. Short-acting preparations should be taken before such predictable episodes. Although less common, patients may have “end-of-dose failure” with long-acting opioids, meaning that they develop pain after 8 h in the case of an every-12-h medication. In these cases, a trial of giving an every-12-h medication every 8 h is appropriate.
Because of differences in opioid receptors, cross-tolerance among opioids is incomplete, and patients may experience different side effects with different opioids. Therefore, if a patient is not experiencing pain relief or is experiencing too many side effects, a change to another opioid preparation is appropriate. When switching, one should begin with 50–75% of the published equianalgesic dose of the new opioid.
Unlike NSAIDs, opioids have no ceiling effect; therefore, there is no maximum dose no matter how many milligrams the patient is receiving. The appropriate dose is the dose needed to achieve pain relief. This is an important point for clinicians to explain to patients and families. Addiction or excessive respiratory depression is extremely unlikely in the terminally ill; fear of these side effects should neither prevent escalating opioid medications when the patient is experiencing insufficient pain relief nor justify using opioid antagonists.
Opioid side effects should be anticipated and treated preemptively. Nearly all patients experience constipation that can be debilitating (see below). Failure to prevent constipation often results in noncompliance with opioid therapy. Methylnaltrexone is a drug that targets opioid-induced constipation by blocking peripheral opioid receptors but not central receptors for analgesia. In placebo-controlled trials, it has been shown to cause laxation within 24 h of administration. As with the use of opioids, about a third of patients using methylnaltrexone experience nausea and vomiting, but unlike constipation, tolerance develops, usually within a week. Therefore, when one is beginning opioids, an antiemetic such as metoclopramide or a serotonin antagonist often is prescribed prophylactically and stopped after 1 week. Olanzapine also has antinausea properties and can be effective in countering delirium or anxiety, with the advantage of some weight gain.
Drowsiness, a common side effect of opioids, also usually abates within a week. During this period, drowsiness can be treated with psychostimulants such as dextroamphetamine, methylphenidate, and modafinil. Modafinil has the advantage of everyday dosing. Pilot reports suggest that donepezil may also be helpful for opiate-induced drowsiness as well as relieving fatigue and anxiety. Metabolites of morphine and most opioids are cleared renally; doses may have to be adjusted for patients with renal failure.
Seriously ill patients who require chronic pain relief rarely if ever become addicted. Suspicion of addiction should not be a reason to withhold pain medications from terminally ill patients. Patients and families may withhold prescribed opioids for fear of addiction or dependence. Physicians and health care providers should reassure patients and families that the patient will not become addicted to opioids if they are used as prescribed for pain relief; this fear should not prevent the patient from taking the medications around the clock. However, diversion of drugs for use by other family members or illicit sale may occur. It may be necessary to advise the patient and caregiver about secure storage of opioids. Contract writing with the patient and family can help. If that fails, transfer to a safe facility may be necessary.
Tolerance is the need to increase medication dosage for the same pain relief without a change in disease. In the case of patients with advanced disease, the need for increasing opioid dosage for pain relief usually is caused by disease progression rather than tolerance. Physical dependence is indicated by symptoms from the abrupt withdrawal of opioids and should not be confused with addiction.
Adjuvant analgesic medications are nonopioids that potentiate the analgesic effects of opioids. They are especially important in the management of neuropathic pain. Gabapentin and pregabalin, calcium channel alpha 2-delta ligands, are now the first-line treatments for neuropathic pain from a variety of causes. Gabapentin is begun at 100–300 mg bid or tid, with 50–100% dose increments every 3 days. Usually 900–3600 mg/d in two or three doses is effective. The combination of gabapentin and nortriptyline may be more effective than gabapentin alone. One potential side effect of gabapentin to be aware of is confusion and drowsiness, especially in the elderly. Pregabalin has the same mechanism of action as gabapentin but is absorbed more efficiently from the GI tract. It is started at 75 mg bid and increased to 150 mg bid. The maximum dose is 225 mg bid. Carbamazepine, a first-generation agent, has been proved effective in randomized trials for neuropathic pain. Other potentially effective anticonvulsant adjuvants include topiramate (25–50 mg qd or bid, rising to 100–300 mg/d) and oxcarbazepine (75–300 mg bid, rising to 1200 mg bid). Glucocorticoids, preferably dexamethasone given once a day, can be useful in reducing inflammation that causes pain while elevating mood, energy, and appetite. Its main side effects include confusion, sleep difficulties, and fluid retention. Glucocorticoids are especially effective for bone pain and abdominal pain from distention of the GI tract or liver. Other drugs, including clonidine and baclofen, can be effective in pain relief. These drugs are adjuvants and generally should be used in conjunction with—not instead of—opioids. Methadone, carefully dosed because of its unpredictable half-life in many patients, has activity at the N-methyl-D-aspartamate (NMDA) receptor and is useful for complex pain syndromes and neuropathic pain. It generally is reserved for cases in which first-line opioids (morphine, oxycodone, hydromorphone) are either ineffective or unavailable.
Radiation therapy can treat bone pain from single metastatic lesions. Bone pain from multiple metastases can be amenable to radiopharmaceuticals such as strontium-89 and samarium-153. Bisphosphonates (such as pamidronate [90 mg every 4 weeks]) and calcitonin (200 IU intranasally once or twice a day) also provide relief from bone pain but have an onset of action of days.
Constipation • FREQUENCY Constipation is reported in up to 87% of patients requiring palliative care.
ETIOLOGY Although hypercalcemia and other factors can cause constipation, it is most frequently a predictable consequence of the use of opioids for the relief of pain and dyspnea and of tricyclic antidepressants, from their anticholinergic effects, and of the inactivity and poor diet that are common among seriously ill patients. If untreated, constipation can cause substantial pain and vomiting and also is associated with confusion and delirium. Whenever opioids and other medications known to cause constipation are used, preemptive treatment for constipation should be instituted.
ASSESSMENT The physician should establish the patient’s previous bowel habits, including the frequency, consistency, and volume. Abdominal and rectal examinations should be performed to exclude impaction or acute abdomen. A number of constipation assessment scales are available, although guidelines issued in the Journal of Palliative Medicine did not recommend them for routine practice. Radiographic assessments beyond a simple flat plate of the abdomen in cases in which obstruction is suspected are rarely necessary.
INTERVENTION Intervention to reestablish comfortable bowel habits and relieve pain and discomfort should be the goals of any measures to address constipation during end-of-life care. Although physical activity, adequate hydration, and dietary treatments with fiber can be helpful, each is limited in its effectiveness for most seriously ill patients, and fiber may exacerbate problems in the setting of dehydration and if impaired motility is the etiology. Fiber is contraindicated in the presence of opioid use. Stimulant and osmotic laxatives, stool softeners, fluids, and enemas are the mainstays of therapy (Table 10-5). In preventing constipation from opioids and other medications, a combination of a laxative and a stool softener (such as senna and docusate) should be used. If after several days of treatment, a bowel movement has not occurred, a rectal examination to remove impacted stool and place a suppository is necessary. For patients with impending bowel obstruction or gastric stasis, octreotide to reduce secretions can be helpful. For patients in whom the suspected mechanism is dysmotility, metoclopramide can be helpful.
MEDICATIONS FOR THE MANAGEMENT OF CONSTIPATION |
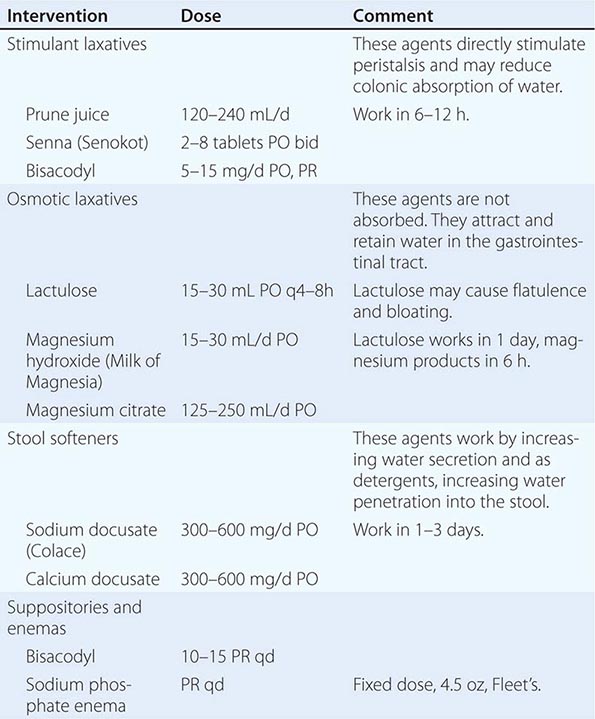
Nausea • FREQUENCY Up to 70% of patients with advanced cancer have nausea, defined as the subjective sensation of wanting to vomit.
ETIOLOGY Nausea and vomiting are both caused by stimulation at one of four sites: the GI tract, the vestibular system, the chemoreceptor trigger zone (CTZ), and the cerebral cortex. Medical treatments for nausea are aimed at receptors at each of these sites: the GI tract contains mechanoreceptors, chemoreceptors, and 5-hydroxytryptamine type 3 (5-HT3) receptors; the vestibular system probably contains histamine and acetylcholine receptors; and the CTZ contains chemoreceptors, dopamine type 2 receptors, and 5-HT3 receptors. An example of nausea that most likely is mediated by the cortex is anticipatory nausea before a dose of chemotherapy or other noxious stimuli.
Specific causes of nausea include metabolic changes (liver failure, uremia from renal failure, hypercalcemia), bowel obstruction, constipation, infection, GERD, vestibular disease, brain metastases, medications (including antibiotics, NSAIDs, proton pump inhibitors, opioids, and chemotherapy), and radiation therapy. Anxiety can also contribute to nausea.
INTERVENTION Medical treatment of nausea is directed at the anatomic and receptor-mediated cause that a careful history and physical examination reveals. When a single specific cause is not found, many advocate beginning treatment with a dopamine antagonist such as haloperidol or prochlorperazine. Prochlorperazine is usually more sedating than haloperidol. When decreased motility is suspected, metoclopramide can be an effective treatment. When inflammation of the GI tract is suspected, glucocorticoids such as dexamethasone are an appropriate treatment. For nausea that follows chemotherapy and radiation therapy, one of the 5-HT3 receptor antagonists (ondansetron, granisetron, dolasetron, palonosetron) is recommended. Studies suggest palonosetron has higher receptor binding affinity and clinical superiority to the other 5-HT3 receptor antagonists. Clinicians should attempt prevention of postchemotherapy nausea rather than provide treatment after the fact. Current clinical guidelines recommend tailoring the strength of treatments to the specific emetic risk posed by a specific chemotherapy drug. When a vestibular cause (such as “motion sickness” or labyrinthitis) is suspected, antihistamines such as meclizine (whose primary side effect is drowsiness) or anticholinergics such as scopolamine can be effective. In anticipatory nausea, a benzodiazepine such as lorazepam is indicated. As with antihistamines, drowsiness and confusion are the main side effects.
Dyspnea • FREQUENCY Dyspnea is a subjective experience of being short of breath. Frequencies vary among causes of death, but it can affect 80–90% of dying patients with lung cancer, COPD, and heart disease. Dyspnea is among the most distressing physical symptoms and can be even more distressing than pain.
ASSESSMENT As with pain, dyspnea is a subjective experience that may not correlate with objective measures of PO2, PCO2, or respiratory rate. Consequently, measurements of oxygen saturation through pulse oximetry or blood gases are rarely helpful in guiding therapy. Despite the limitations of existing assessment methods, physicians should regularly assess and document patients’ experience of dyspnea and its intensity. Guidelines recommend visual or analogue dyspnea scales to assess the severity of symptoms and the effects of treatment. Potentially reversible or treatable causes of dyspnea include infection, pleural effusions, pulmonary emboli, pulmonary edema, asthma, and tumor encroachment on the airway. However, the risk-versus-benefit ratio of the diagnostic and therapeutic interventions for patients with little time left to live must be considered carefully before one undertakes diagnostic steps. Frequently, the specific etiology cannot be identified, and dyspnea is the consequence of progression of the underlying disease that cannot be treated. The anxiety caused by dyspnea and the choking sensation can significantly exacerbate the underlying dyspnea in a negatively reinforcing cycle.
INTERVENTIONS When reversible or treatable etiologies are diagnosed, they should be treated as long as the side effects of treatment, such as repeated drainage of effusions or anticoagulants, are less burdensome than the dyspnea itself. More aggressive treatments such as stenting a bronchial lesion may be warranted if it is clear that the dyspnea is due to tumor invasion at that site and if the patient and family understand the risks of such a procedure. Usually, treatment will be symptomatic (Table 10-6). A dyspnea scale and careful monitoring should guide dose adjustment. Low-dose opioids reduce the sensitivity of the central respiratory center and the sensation of dyspnea. If patients are not receiving opioids, weak opioids can be initiated; if patients are already receiving opioids, morphine or other strong opioids should be used. Controlled trials do not support the use of nebulized opioids for dyspnea at the end of life. Phenothiazines and chlorpromazine may be helpful when combined with opioids. Benzodiazepines can be helpful if anxiety is present but should be neither used as first-line therapy nor used alone in the treatment of dyspnea. If the patient has a history of COPD or asthma, inhaled bronchodilators and glucocorticoids may be helpful. If the patient has pulmonary edema due to heart failure, diuresis with a medication such as furosemide is indicated. Excess secretions can be dried with scopolamine, transdermally or intravenously. Use of oxygen is controversial. There are conflicting data on its effectiveness for patients with proven hypoxemia. But there is no clear benefit of oxygen compared to room air for nonhypoxemic patients. Noninvasive positive-pressure ventilation using a facemask or nasal plugs may be used for some patients for symptom relief. For some families and patients, oxygen is distressing; for others, it is reassuring. More general interventions that medical staff can do include sitting the patient upright, removing smoke or other irritants such as perfume, ensuring a supply of fresh air with sufficient humidity, and minimizing other factors that can increase anxiety.
MEDICATIONS FOR THE MANAGEMENT OF DYSPNEA |
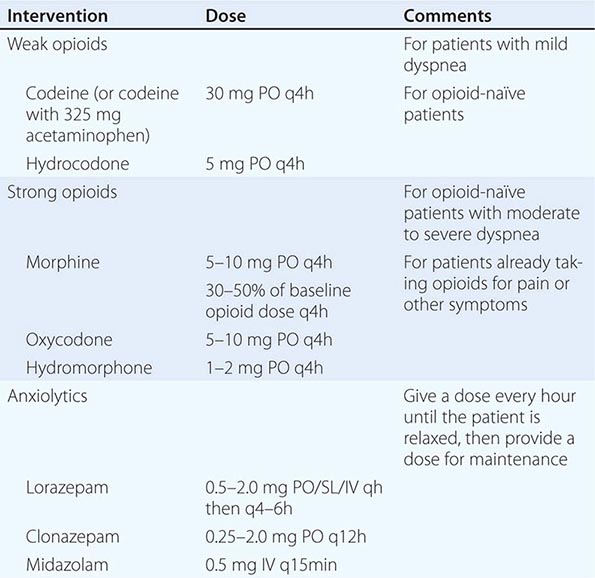
Fatigue • FREQUENCY More than 90% of terminally ill patients experience fatigue and/or weakness. Fatigue is one of the most commonly reported symptoms of cancer treatment as well as in the palliative care of multiple sclerosis, COPD, heart failure, and HIV. Fatigue frequently is cited as among the most distressing symptoms.
ETIOLOGY The multiple causes of fatigue in the terminally ill can be categorized as resulting from the underlying disease; from disease-induced factors such as tumor necrosis factor and other cytokines; and from secondary factors such as dehydration, anemia, infection, hypothyroidism, and drug side effects. Apart from low caloric intake, loss of muscle mass and changes in muscle enzymes may play an important role in fatigue of terminal illness. The importance of changes in the CNS, especially the reticular activating system, have been hypothesized based on reports of fatigue in patients receiving cranial radiation, experiencing depression, or having chronic pain in the absence of cachexia or other physiologic changes. Finally, depression and other causes of psychological distress can contribute to fatigue.
ASSESSMENT Like pain and dyspnea, fatigue is subjective. Objective changes, even in body mass, may be absent. Consequently, assessment must rely on patient self-reporting. Scales used to measure fatigue, such as the Edmonton Functional Assessment Tool, the Fatigue Self-Report Scales, and the Rhoten Fatigue Scale, are usually appropriate for research rather than clinical purposes. In clinical practice, a simple performance assessment such as the Karnofsky Performance Status or the ECOG’s question “How much of the day does the patient spend in bed?” may be the best measure. In this 0–4 performance status assessment, 0 = normal activity; 1 = symptomatic without being bedridden; 2 = requiring some, but <50%, bed time; 3 = bedbound more than half the day; and 4 = bedbound all the time. Such a scale allows for assessment over time and correlates with overall disease severity and prognosis. A 2008 review by the European Association of Palliative Care also described several longer assessment tools with 9–20 items, including the Piper Fatigue Inventory, the Multidimensional Fatigue Inventory, and the Brief Fatigue Inventory (BFI).
INTERVENTIONS For some patients, there are reversible causes such as anemia, but for most patients at the end of life, fatigue will not be “cured.” The goal is to ameliorate it and help patients and families adjust expectations. Behavioral interventions should be used to avoid blaming the patient for inactivity and to educate both the family and the patient that the underlying disease causes physiologic changes that produce low energy levels. Understanding that the problem is physiologic and not psychological can help alter expectations regarding the patient’s level of physical activity. Practically, this may mean reducing routine activities such as housework and cooking or social events outside the house and making it acceptable to receive guests lying on a couch. At the same time, institution of exercise regimens and physical therapy can raise endorphins, reduce muscle wasting, and reduce the risk of depression. In addition, ensuring good hydration without worsening edema may help reduce fatigue. Discontinuing medications that worsen fatigue may help, including cardiac medications, benzodiazepines, certain antidepressants, or opioids if pain is well-controlled. As end-of-life care proceeds into its final stages, fatigue may protect patients from further suffering, and continued treatment could be detrimental.
There are woefully few pharmacologic interventions that target fatigue and weakness. Glucocorticoids can increase energy and enhance mood. Dexamethasone is preferred for its once-a-day dosing and minimal mineralocorticoid activity. Benefit, if any, usually is seen within the first month. Psychostimulants such as dextroamphetamine (5–10 mg PO) and methylphenidate (2.5–5 mg PO) may also enhance energy levels, although a randomized trial did not show methylphenidate beneficial compared with placebo in cancer fatigue. Doses should be given in the morning and at noon to minimize the risk of counterproductive insomnia. Modafinil, developed for narcolepsy, has shown some promise in the treatment of severe fatigue and has the advantage of once-daily dosing. Its precise role in fatigue at the end of life has not been determined. Anecdotal evidence suggests that L-carnitine may improve fatigue, depression, and sleep disruption. Similarly, some studies suggest ginseng can reduce fatigue.
PSYCHOLOGICAL SYMPTOMS AND THEIR MANAGEMENT
Depression • FREQUENCY Depression at the end of life presents an apparently paradoxical situation. Many people believe that depression is normal among seriously ill patients because they are dying. People frequently say, “Wouldn’t you be depressed?” However, depression is not a necessary part of terminal illness and can contribute to needless suffering. Although sadness, anxiety, anger, and irritability are normal responses to a serious condition, they are typically of modest intensity and transient. Persistent sadness and anxiety and the physically disabling symptoms that they can lead to are abnormal and suggestive of major depression. Although as many as 75% of terminally ill patients experience emotional distress and depressive symptoms, <30% of terminally ill patients have major depression. Depression is not limited to cancer patients but found in patients with end-stage renal disease, Parkinson’s disease, multiple sclerosis, and other terminal conditions.
ETIOLOGY Previous history of depression, family history of depression or bipolar disorder, and prior suicide attempts are associated with increased risk for depression among terminally ill patients. Other symptoms, such as pain and fatigue, are associated with higher rates of depression; uncontrolled pain can exacerbate depression, and depression can cause patients to be more distressed by pain. Many medications used in the terminal stages, including glucocorticoids, and some anticancer agents, such as tamoxifen, interleukin 2, interferon α, and vincristine, also are associated with depression. Some terminal conditions, such as pancreatic cancer, certain strokes, and heart failure, have been reported to be associated with higher rates of depression, although this is controversial. Finally, depression may be attributable to grief over the loss of a role or function, social isolation, or loneliness.
ASSESSMENT Diagnosing depression among seriously ill patients is complicated because many of the vegetative symptoms in the DSM-V (Diagnostic and Statistical Manual of Mental Disorders) criteria for clinical depression—insomnia, anorexia and weight loss, fatigue, decreased libido, and difficulty concentrating—are associated with the dying process itself. The assessment of depression in seriously ill patients therefore should focus on the dysphoric mood, helplessness, hopelessness, and lack of interest and enjoyment and concentration in normal activities. The single questions “How often do you feel downhearted and blue?” (more than a good bit of the time or similar responses) and “Do you feel depressed most of the time?” are appropriate for screening. Visual Analog Scales can also be useful in screening.
INTERVENTIONS Physicians must treat any physical symptom, such as pain, that may be causing or exacerbating depression. Fostering adaptation to the many losses that the patient is experiencing can also be helpful. Nonpharmacologic interventions, including group or individual psychological counseling, and behavioral therapies such as relaxation and imagery can be helpful, especially in combination with drug therapy.
Pharmacologic interventions remain the core of therapy. The same medications are used to treat depression in terminally ill as in non–terminally ill patients. Psychostimulants may be preferred for patients with a poor prognosis or for those with fatigue or opioid-induced somnolence. Psychostimulants are comparatively fast acting, working within a few days instead of the weeks required for selective serotonin reuptake inhibitors (SSRIs). Dextroamphetamine or methylphenidate should be started at 2.5–5.0 mg in the morning and at noon, the same starting doses used for treating fatigue. The dose can be escalated up to 15 mg bid. Modafinil is started at 100 mg qd and can be increased to 200 mg if there is no effect at the lower dose. Pemoline is a nonamphetamine psychostimulant with minimal abuse potential. It is also effective as an antidepressant beginning at 18.75 mg in the morning and at noon. Because it can be absorbed through the buccal mucosa, it is preferred for patients with intestinal obstruction or dysphagia. If it is used for prolonged periods, liver function must be monitored. The psychostimulants can also be combined with more traditional antidepressants while waiting for the antidepressants to become effective and then tapered after a few weeks if necessary. Psychostimulants have side effects, particularly initial anxiety, insomnia, and rarely paranoia, which may necessitate lowering the dose or discontinuing treatment.
Mirtazapine, an antagonist at the postsynaptic serotonin receptors, is a promising psychostimulant. It should be started at 7.5 mg before bed. It has sedating, antiemetic, and anxiolytic properties with few drug interactions. Its side effect of weight gain may be beneficial for seriously ill patients; it is available in orally disintegrating tablets.
For patients with a prognosis of several months or longer, SSRIs, including fluoxetine, sertraline, paroxetine, citalopram, escitalopram, and fluvoxamine, and serotonin-noradrenaline reuptake inhibitors such as venlafaxine, are the preferred treatment because of their efficacy and comparatively few side effects. Because low doses of these medications may be effective for seriously ill patients, one should use half the usual starting dose for healthy adults. The starting dose for fluoxetine is 10 mg once a day. In most cases, once-a-day dosing is possible. The choice of which SSRI to use should be driven by (1) the patient’s past success or failure with the specific medication, (2) the most favorable side effect profile for that specific agent, and (3) the time it takes to reach steady-state drug levels. For instance, for a patient in whom fatigue is a major symptom, a more activating SSRI (fluoxetine) would be appropriate. For a patient in whom anxiety and sleeplessness are major symptoms, a more sedating SSRI (paroxetine) would be appropriate.
Atypical antidepressants are recommended only in selected circumstances, usually with the assistance of a specialty consultation. Trazodone can be an effective antidepressant but is sedating and can cause orthostatic hypotension and, rarely, priapism. Therefore, it should be used only when a sedating effect is desired and is often used for patients with insomnia, at a dose starting at 25 mg. In addition to its antidepressant effects, bupropion is energizing, making it useful for depressed patients who experience fatigue. However, it can cause seizures, preventing its use for patients with a risk of CNS neoplasms or terminal delirium. Finally, alprazolam, a benzodiazepine, starting at 0.25–1.0 mg tid, can be effective in seriously ill patients who have a combination of anxiety and depression. Although it is potent and works quickly, it has many drug interactions and may cause delirium, especially among very ill patients, because of its strong binding to the benzodiazepine–γ-aminobutyric acid (GABA) receptor complex.
Unless used as adjuvants for the treatment of pain, tricyclic antidepressants are not recommended. Similarly, monoamine oxidase (MAO) inhibitors are not recommended because of their side effects and dangerous drug interactions.
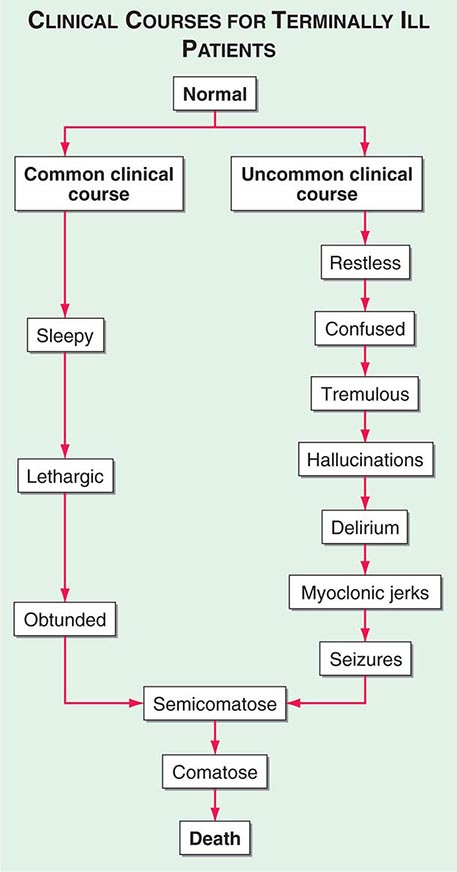
Delirium (See Chap. 34) • FREQUENCY In the weeks or months before death, delirium is uncommon, although it may be significantly underdiagnosed. However, delirium becomes relatively common in the hours and days immediately before death. Up to 85% of patients dying from cancer may experience terminal delirium.
ETIOLOGY Delirium is a global cerebral dysfunction characterized by alterations in cognition and consciousness. It frequently is preceded by anxiety, changes in sleep patterns (especially reversal of day and night), and decreased attention. In contrast to dementia, delirium has an acute onset, is characterized by fluctuating consciousness and inattention, and is reversible, although reversibility may be more theoretical than real for patients near death. Delirium may occur in a patient with dementia; indeed, patients with dementia are more vulnerable to delirium.
Causes of delirium include metabolic encephalopathy arising from liver or renal failure, hypoxemia, or infection; electrolyte imbalances such as hypercalcemia; paraneoplastic syndromes; dehydration; and primary brain tumors, brain metastases, or leptomeningeal spread of tumor. Commonly, among dying patients, delirium can be caused by side effects of treatments, including radiation for brain metastases, and medications, including opioids, glucocorticoids, anticholinergic drugs, antihistamines, antiemetics, benzodiazepines, and chemotherapeutic agents. The etiology may be multifactorial; e.g., dehydration may exacerbate opioid-induced delirium.
ASSESSMENT Delirium should be recognized in any terminally ill patient with new onset of disorientation, impaired cognition, somnolence, fluctuating levels of consciousness, or delusions with or without agitation. Delirium must be distinguished from acute anxiety and depression as well as dementia. The central distinguishing feature is altered consciousness, which usually is not noted in anxiety, depression, and dementia. Although “hyperactive” delirium characterized by overt confusion and agitation is probably more common, patients also should be assessed for “hypoactive” delirium characterized by sleep-wake reversal and decreased alertness.
In some cases, use of formal assessment tools such as the Mini-Mental Status Examination (which does not distinguish delirium from dementia) and the Delirium Rating Scale (which does distinguish delirium from dementia) may be helpful in distinguishing delirium from other processes. The patient’s list of medications must be evaluated carefully. Nonetheless, a reversible etiologic factor for delirium is found in fewer than half of terminally ill patients. Because most terminally ill patients experiencing delirium will be very close to death and may be at home, extensive diagnostic evaluations such as lumbar punctures and neuroradiologic examinations are usually inappropriate.
INTERVENTIONS One of the most important objectives of terminal care is to provide terminally ill patients the lucidity to say goodbye to the people they love. Delirium, especially with agitation during the final days, is distressing to family and caregivers. A strong determinant of bereavement difficulties is witnessing a difficult death. Thus, terminal delirium should be treated aggressively.
At the first sign of delirium, such as day-night reversal with slight changes in mentation, the physician should let the family members know that it is time to be sure that everything they want to say has been said. The family should be informed that delirium is common just before death.
If medications are suspected of being a cause of the delirium, unnecessary agents should be discontinued. Other potentially reversible causes, such as constipation, urinary retention, and metabolic abnormalities, should be treated. Supportive measures aimed at providing a familiar environment should be instituted, including restricting visits only to individuals with whom the patient is familiar and eliminating new experiences; orienting the patient, if possible, by providing a clock and calendar; and gently correcting the patient’s hallucinations or cognitive mistakes.
Pharmacologic management focuses on the use of neuroleptics and, in the extreme, anesthetics (Table 10-7). Haloperidol remains first-line therapy. Usually, patients can be controlled with a low dose (1–3 mg/d), usually given every 6 h, although some may require as much as 20 mg/d. It can be administered PO, SC, or IV. IM injections should not be used, except when this is the only way to get a patient under control. Newer atypical neuroleptics, such as olanzapine, risperidone, and quetiapine, have shown significant effectiveness in completely resolving delirium in cancer patients. These drugs also have fewer side effects than haloperidol, along with other beneficial effects for terminally ill patients, including antinausea, antianxiety, and weight gain. They are useful for patients with longer anticipated life expectancy because they are less likely to cause dysphoria and have a lower risk of dystonic reactions. Also, because they are metabolized through multiple pathways, they can be used in patients with hepatic and renal dysfunction. Olanzapine has the disadvantage that it is available only orally and that it takes a week to reach steady state. The usual dose is 2.5–5 mg PO bid. Chlorpromazine (10–25 mg every 4–6 h) can be useful if sedation is desired and can be administered IV or PR in addition to PO. Dystonic reactions resulting from dopamine blockade are a side effect of neuroleptics, although they are reported to be rare when these drugs are used to treat terminal delirium. If patients develop dystonic reactions, benztropine should be administered. Neuroleptics may be combined with lorazepam to reduce agitation when the delirium is the result of alcohol or sedative withdrawal.
MEDICATIONS FOR THE MANAGEMENT OF DELIRIUM |
If no response to first-line therapy is seen, a specialty consultation should be obtained with a change to a different medication. If patients fail to improve after a second neuroleptic, sedation with an anesthetic such as propofol or continuous-infusion midazolam may be necessary. By some estimates, at the very end of life, as many as 25% of patients experiencing delirium, especially restless delirium with myoclonus or convulsions, may require sedation.
Physical restraints should be used with great reluctance and only when the patient’s violence is threatening to self or others. If they are used, their appropriateness should be reevaluated frequently.
Insomnia • FREQUENCY Sleep disorders, defined as difficulty initiating sleep or maintaining sleep, sleep difficulty at least 3 nights a week, or sleep difficulty that causes impairment of daytime functioning, occur in 19–63% of patients with advanced cancer. Some 30–74% of patients with other end-stage conditions, including AIDS, heart disease, COPD, and renal disease, experience insomnia.
ETIOLOGY Patients with cancer may have changes in sleep efficiency such as an increase in stage I sleep. Other etiologies of insomnia are coexisting physical illness such as thyroid disease and coexisting psychological illnesses such as depression and anxiety. Medications, including antidepressants, psychostimulants, steroids, and β agonists, are significant contributors to sleep disorders, as are caffeine and alcohol. Multiple over-the-counter medications contain caffeine and antihistamines, which can contribute to sleep disorders.
ASSESSMENT Assessment should include specific questions concerning sleep onset, sleep maintenance, and early-morning wakening as these will provide clues to the causative agents and to management. Patients should be asked about previous sleep problems, screened for depression and anxiety, and asked about symptoms of thyroid disease. Caffeine and alcohol are prominent causes of sleep problems, and a careful history of the use of these substances should be obtained. Both excessive use and withdrawal from alcohol can be causes of sleep problems.
INTERVENTIONS The mainstays of intervention include improvement of sleep hygiene (encouragement of regular time for sleep, decreased nighttime distractions, elimination of caffeine and other stimulants and alcohol), intervention to treat anxiety and depression, and treatment for the insomnia itself. For patients with depression who have insomnia and anxiety, a sedating antidepressant such as mirtazapine can be helpful. In the elderly, trazodone, beginning at 25 mg at nighttime, is an effective sleep aid at doses lower than those which cause its antidepressant effect. Zolpidem may have a decreased incidence of delirium in patients compared with traditional benzodiazepines, but this has not been clearly established. When benzodiazepines are prescribed, short-acting ones (such as lorazepam) are favored over longer-acting ones (such as diazepam). Patients who receive these medications should be observed for signs of increased confusion and delirium.
SOCIAL NEEDS AND THEIR MANAGEMENT
Financial Burdens • FREQUENCY Dying can impose substantial economic strains on patients and families, causing distress. In the United States, with one of the least comprehensive health insurance systems among the developed countries, ~20% of terminally ill patients and their families spend >10% of family income on health care costs over and above health insurance premiums. Between 10 and 30% of families sell assets, use savings, or take out a mortgage to pay for the patient’s health care costs. Nearly 40% of terminally ill patients in the United States report that the cost of their illness is a moderate or great economic hardship for their family.
The patient is likely to reduce and eventually stop working. In 20% of cases, a family member of the terminally ill patient also stops working to provide care. The major underlying causes of economic burden are related to poor physical functioning and care needs, such as the need for housekeeping, nursing, and personal care. More debilitated patients and poor patients experience greater economic burdens.
INTERVENTION This economic burden should not be ignored as a private matter. It has been associated with a number of adverse health outcomes, including preferring comfort care over life-prolonging care as well as consideration of euthanasia or physician-assisted suicide. Economic burdens increase the psychological distress of families and caregivers of terminally ill patients, and poverty is associated with many adverse health outcomes. Importantly, recent studies found that “patients with advanced cancer who reported having end-of-life conversations with physicians had significantly lower health care costs in their final week of life. Higher costs were associated with worse quality of death.” Assistance from a social worker, early on if possible, to ensure access to all available benefits may be helpful. Many patients, families, and health care providers are unaware of options for long-term care insurance, respite care, the Family Medical Leave Act (FMLA), and other sources of assistance. Some of these options (such as respite care) may be part of a formal hospice program, but others (such as the FMLA) do not require enrollment in a hospice program.
Relationships • FREQUENCY Settling personal issues and closing the narrative of lived relationships are universal needs. When asked if sudden death or death after an illness is preferable, respondents often initially select the former but soon change to the latter as they reflect on the importance of saying goodbye. Bereaved family members who have not had the chance to say goodbye often have a more difficult grief process.
INTERVENTIONS Care of seriously ill patients requires efforts to facilitate the types of encounters and time spent with family and friends that are necessary to meet those needs. Family and close friends may need to be accommodated with unrestricted visiting hours, which may include sleeping near the patient even in otherwise regimented institutional settings. Physicians and other health care providers may be able to facilitate and resolve strained interactions between the patient and other family members. Assistance for patients and family members who are unsure about how to create or help preserve memories, whether by providing materials such as a scrapbook or memory box or by offering them suggestions and informational resources, can be deeply appreciated. Taking photographs and creating videos can be especially helpful to terminally ill patients who have younger children or grandchildren.
Family Caregivers • FREQUENCY Caring for seriously ill patients places a heavy burden on families. Families frequently are required to provide transportation and homemaking as well as other services. Typically, paid professionals such as home health nurses and hospice workers supplement family care; only about a quarter of all caregiving consists of exclusively paid professional assistance. The trend toward more out-of-hospital deaths will increase reliance on families for end-of-life care. Increasingly, family members are being called upon to provide physical care (such as moving and bathing patients) and medical care (such as assessing symptoms and giving medications) in addition to emotional care and support.
Three-quarters of family caregivers of terminally ill patients are women—wives, daughters, sisters, and even daughters-in-law. Because many are widowed, women tend to be able to rely less on family for caregiving assistance and may need more paid assistance. About 20% of terminally ill patients report substantial unmet needs for nursing and personal care. The impact of caregiving on family caregivers is substantial: both bereaved and current caregivers have a higher mortality rate than that of non-caregiving controls.
INTERVENTIONS It is imperative to inquire about unmet needs and to try to ensure that those needs are met either through the family or by paid professional services when possible. Community assistance through houses of worship or other community groups often can be mobilized by telephone calls from the medical team to someone the patient or family identifies. Sources of support specifically for family caregivers should be identified through local sources or nationally through groups such as the National Family Caregivers Association (www.nfcacares.org), the American Cancer Society (www.cancer.org), and the Alzheimer’s Association (www.alz.org).
EXISTENTIAL NEEDS AND THEIR MANAGEMENT
Frequency Religion and spirituality are often important to dying patients. Nearly 70% of patients report becoming more religious or spiritual when they became terminally ill, and many find comfort in religious or spiritual practices such as prayer. However, ~20% of terminally ill patients become less religious, frequently feeling cheated or betrayed by becoming terminally ill. For other patients, the need is for existential meaning and purpose that is distinct from and may even be antithetical to religion or spirituality. When asked, patients and family caregivers frequently report wanting their professional caregivers to be more attentive to religion and spirituality.
ASSESSMENT Health care providers are often hesitant about involving themselves in the religious, spiritual, and existential experiences of their patients because it may seem private or not relevant to the current illness. But physicians and other members of the care team should be able at least to detect spiritual and existential needs. Screening questions have been developed for a physician’s spiritual history taking. Spiritual distress can amplify other types of suffering and even masquerade as intractable physical pain, anxiety, or depression. The screening questions in the comprehensive assessment are usually sufficient. Deeper evaluation and intervention are rarely appropriate for the physician unless no other member of a care team is available or suitable. Pastoral care providers may be helpful, whether from the medical institution or from the patient’s own community.
INTERVENTIONS Precisely how religious practices, spirituality, and existential explorations can be facilitated and improve end-of-life care is not well established. What is clear is that for physicians, one main intervention is to inquire about the role and importance of spirituality and religion in a patient’s life. This will help a patient feel heard and help physicians identify specific needs. In one study, only 36% of respondents indicated that a clergy member would be comforting. Nevertheless, the increase in religious and spiritual interest among a substantial fraction of dying patients suggests inquiring of individual patients how this need can be addressed. Some evidence supports specific methods of addressing existential needs in patients, ranging from establishing a supportive group environment for terminal patients to individual treatments emphasizing a patient’s dignity and sources of meaning.
MANAGING THE LAST STAGES
WITHDRAWING AND WITHHOLDING LIFE-SUSTAINING TREATMENT
LEGAL ASPECTS For centuries, it has been deemed ethical to withhold or withdraw life-sustaining interventions. The current legal consensus in the United States and most developed countries is that patients have a moral as well as constitutional or common law right to refuse medical interventions. American courts also have held that incompetent patients have a right to refuse medical interventions. For patients who are incompetent and terminally ill and who have not completed an advance care directive, next of kin can exercise that right, although this may be restricted in some states, depending how clear and convincing the evidence is of the patient’s preferences. Courts have limited families’ ability to terminate life-sustaining treatments in patients who are conscious, incompetent, but not terminally ill. In theory, patients’ right to refuse medical therapy can be limited by four countervailing interests: (1) preservation of life, (2) prevention of suicide, (3) protection of third parties such as children, and (4) preservation of the integrity of the medical profession. In practice, these interests almost never override the right of competent patients and incompetent patients who have left explicit and advance care directives.
For incompetent patients who either appointed a proxy without specific indications of their wishes or never completed an advance care directive, three criteria have been suggested to guide the decision to terminate medical interventions. First, some commentators suggest that ordinary care should be administered but extraordinary care could be terminated. Because the ordinary/extraordinary distinction is too vague, courts and commentators widely agree that it should not be used to justify decisions about stopping treatment. Second, many courts have advocated the use of the substituted-judgment criterion, which holds that the proxy decision-makers should try to imagine what the incompetent patient would do if he or she were competent. However, multiple studies indicate that many proxies, even close family members, cannot accurately predict what the patient would have wanted. Therefore, substituted judgment becomes more of a guessing game than a way of fulfilling the patient’s wishes. Finally, the best-interests criterion holds that proxies should evaluate treatments by balancing their benefits and risks and select those treatments in which the benefits maximally outweigh the burdens of treatment. Clinicians have a clear and crucial role in this by carefully and dispassionately explaining the known benefits and burdens of specific treatments. Yet even when that information is as clear as possible, different individuals can have very different views of what is in the patient’s best interests, and families may have disagreements or even overt conflicts. This criterion has been criticized because there is no single way to determine the balance between benefits and burdens; it depends on a patient’s personal values. For instance, for some people, being alive even if mentally incapacitated is a benefit, whereas for others, it may be the worst possible existence. As a matter of practice, physicians rely on family members to make decisions that they feel are best and object only if those decisions seem to demand treatments that the physicians consider not beneficial.
PRACTICES Withholding and withdrawing acutely life-sustaining medical interventions from terminally ill patients are now standard practice. More than 90% of American patients die without cardiopulmonary resuscitation (CPR), and just as many forgo other potentially life-sustaining interventions. For instance, in ICUs in the period 1987–1988, CPR was performed 49% of the time, but it was performed only 10% of the time in 1992–1993. On average, 3.8 interventions, such as vasopressors and transfusions, were stopped for each dying ICU patient. However, up to 19% of decedents in hospitals received interventions such as extubation, ventilation, and surgery in the 48 h preceding death. However, practices vary widely among hospitals and ICUs, suggesting an important element of physician preferences rather than objective data.
Mechanical ventilation may be the most challenging intervention to withdraw. The two approaches are terminal extubation, which is the removal of the endotracheal tube, and terminal weaning, which is the gradual reduction of the or ventilator rate. One-third of ICU physicians prefer to use the terminal weaning technique, and 13% extubate; the majority of physicians use both techniques. The American Thoracic Society’s 2008 clinical policy guidelines note that there is no single correct process of ventilator withdrawal and that physicians use and should be proficient in both methods but that the chosen approach should carefully balance benefits and burdens as well as patient and caregiver preferences. Physicians’ assessment of patients’ likelihood of survival, their prediction of possible cognitive damage, and patients’ preferences about the use of life support are primary factors in determining the likelihood of withdrawal of mechanical ventilation. Some recommend terminal weaning because patients do not develop upper airway obstruction and the distress caused by secretions or stridor; however, terminal weaning can prolong the dying process and not allow a patient’s family to be with him or her unencumbered by an endotracheal tube. To ensure comfort for conscious or semiconscious patients before withdrawal of the ventilator, neuromuscular blocking agents should be terminated and sedatives and analgesics administered. Removing the neuromuscular blocking agents permits patients to show discomfort, facilitating the titration of sedatives and analgesics; it also permits interactions between patients and their families. A common practice is to inject a bolus of midazolam (2–4 mg) or lorazepam (2–4 mg) before withdrawal, followed by 5–10 mg of morphine and continuous infusion of morphine (50% of the bolus dose per hour) during weaning. In patients who have significant upper airway secretions, IV scopolamine at a rate of 100 μg/h can be administered. Additional boluses of morphine or increases in the infusion rate should be administered for respiratory distress or signs of pain. Higher doses will be needed for patients already receiving sedatives and opioids. Families need to be reassured about treatments for common symptoms after withdrawal of ventilatory support, such as dyspnea and agitation, and warned about the uncertainty of length of survival after withdrawal of ventilatory support: up to 10% of patients unexpectedly survive for 1 day or more after mechanical ventilation is stopped.
FUTILE CARE
Beginning in the late 1980s, some commentators argued that physicians could terminate futile treatments demanded by the families of terminally ill patients. Although no objective definition or standard of futility exists, several categories have been proposed. Physiologic futility means that an intervention will have no physiologic effect. Some have defined qualitative futility as applying to procedures that “fail to end a patient’s total dependence on intensive medical care.” Quantitative futility occurs “when physicians conclude (through personal experience, experiences shared with colleagues, or consideration of reported empiric data) that in the last 100 cases, a medical treatment has been useless.” The term conceals subjective value judgments about when a treatment is “not beneficial.” Deciding whether a treatment that obtains an additional 6 weeks of life or a 1% survival advantage confers benefit depends on patients’ preferences and goals. Furthermore, physicians’ predictions of when treatments were futile deviated markedly from the quantitative definition. When residents thought CPR was quantitatively futile, more than one in five patients had a >10% chance of survival to hospital discharge. Most studies that purport to guide determinations of futility are based on insufficient data to provide statistical confidence for clinical decision making. Quantitative futility rarely applies in ICU settings. Many commentators reject using futility as a criterion for withdrawing care, preferring instead to consider futility situations as ones that represent conflict that calls for careful negotiation between families and health care providers.
In the wake of a lack of consensus over quantitative measures of futility, many hospitals adopted process-based approaches to resolve disputes over futility and enhance communication with patients and surrogates, including focusing on interests and alternatives rather than opposing positions and generating a wide range of options. Some hospitals have enacted “unilateral do not resuscitate (DNR)” policies to allow clinicians to provide a DNR order in cases in which consensus cannot be reached with families and medical opinion is that resuscitation would be futile if attempted. This type of a policy is not a replacement for careful and patient communication and negotiation but recognizes that agreement cannot always be reached. Over the last 15 years, many states, such as Texas, Virginia, Maryland, and California, have enacted so-called medical futility laws that provide physicians a “safe harbor” from liability if they refuse a patient or family’s request for life-sustaining interventions. For instance, in Texas when a disagreement about terminating interventions between the medical team and the family has not been resolved by an ethics consultation, the hospital is supposed to try to facilitate transfer of the patient to an institution willing to provide treatment. If this fails after 10 days, the hospital and physician may unilaterally withdraw treatments determined to be futile. The family may appeal to a state court. Early data suggest that the law increases futility consultations for the ethics committee and that although most families concur with withdrawal, about 10–15% of families refuse to withdraw treatment. Approximately 12 cases have gone to court in Texas in the 7 years since the adoption of the law. As of 2007, there had been 974 ethics committee consultations on medical futility cases and 65 in which committees ruled against families and gave notice that treatment would be terminated. Treatment was withdrawn for 27 of those patients, and the remainder were transferred to other facilities or died while awaiting transfer.
EUTHANASIA AND PHYSICIAN-ASSISTED SUICIDE
Euthanasia and physician-assisted suicide are defined in Table 10-8. Terminating life-sustaining care and providing opioid medications to manage symptoms have long been considered ethical by the medical profession and legal by courts and should not be confused with euthanasia or physician-assisted suicide.
DEFINITIONS OF ASSISTED SUICIDE AND EUTHANASIA |
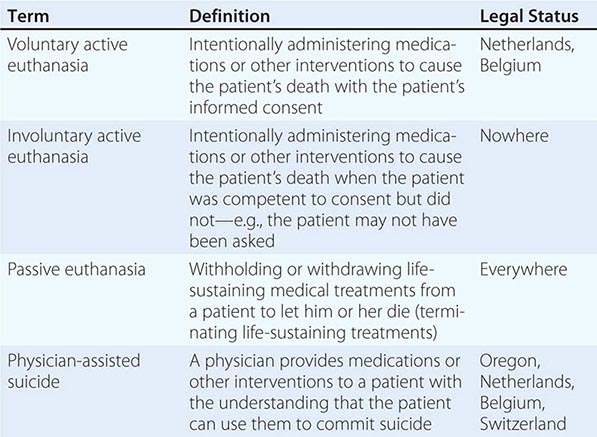
LEGAL ASPECTS Euthanasia is legal in the Netherlands, Belgium, and Luxembourg. It was legalized in the Northern Territory of Australia in 1995, but that legislation was repealed in 1997. Euthanasia is not legal in any state in the United States. With certain conditions, in Switzerland, a layperson can legally assist suicide. In the United States, physician-assisted suicide is legal in four states: Oregon, Vermont, and Washington State by legislation and Montana by court ruling. In jurisdictions where physician-assisted suicide is legal, physicians wishing to prescribe the necessary medication must fulfill multiple criteria and complete processes that include a waiting period. In other countries and all other states in the United States, physician-assisted suicide and euthanasia are illegal explicitly or by common law.
PRACTICES Fewer than 10–20% of terminally ill patients actually consider euthanasia and/or physician-assisted suicide for themselves. In the Netherlands and Oregon, >70% of patients using these interventions are dying of cancer; in Oregon, in 2013, just 1.2% of physician-assisted suicide cases involved patients with HIV/AIDS and 7.2% involved patients with amyotrophic lateral sclerosis. In the Netherlands, the share of deaths attributable to euthanasia or physician-assisted suicide declined from around 2.8% of all deaths in 2001 to around 1.8% in 2005. In 2013, the last year with complete data, around 71 patients in Oregon (just 0.2% of all deaths) died by physician-assisted suicide, although this may be an underestimate. In Washington State, between March 2009 (when the law allowing physician-assisted suicide went into force) and December 2009, 36 individuals died from prescribed lethal doses.
Pain is not a primary motivator for patients’ requests for or interest in euthanasia and/or physician-assisted suicide. Fewer than 25% of all patients in Oregon cite inadequate pain control as the reason for desiring physician-assisted suicide. Depression, hopelessness, and, more profoundly, concerns about loss of dignity or autonomy or being a burden on family members appear to be primary factors motivating a desire for euthanasia or physician-assisted suicide. Over 75% cite loss of autonomy or dignity and inability to engage in enjoyable activities as the reason for wanting physician-assisted suicide. About 40% cite being a burden on family. A study from the Netherlands showed that depressed terminally ill cancer patients were four times more likely to request euthanasia and confirmed that uncontrolled pain was not associated with greater interest in euthanasia. Interestingly, despite the importance of emotional distress in motivating requests for euthanasia and physician-assisted suicide, few patients receive psychiatric care. For instance, in Oregon, only 5.9% of patients have been referred for psychiatric evaluation.
Euthanasia and physician-assisted suicide are no guarantee of a painless, quick death. Data from the Netherlands indicate that in as many as 20% of cases technical and other problems arose, including patients waking from coma, not becoming comatose, regurgitating medications, and experiencing a prolonged time to death. Data from Oregon indicate that between 1997 and 2013, 22 patients (~5%) regurgitated after taking prescribed medication, 1 patient awaked, and none experienced seizures. Problems were significantly more common in physician-assisted suicide, sometimes requiring the physician to intervene and provide euthanasia.
Whether practicing in a setting where euthanasia is legal or not, over a career, 12–54% of physicians receive a request for euthanasia or physician-assisted suicide from a patient. Competency in dealing with such a request is crucial. Although challenging, the request can also provide a chance to address intense suffering. After receiving a request for euthanasia and/or physician-assisted suicide, health care providers should carefully clarify the request with empathic, open-ended questions to help elucidate the underlying cause for the request, such as “What makes you want to consider this option?” Endorsing either moral opposition or moral support for the act tends to be counterproductive, giving an impression of being judgmental or of endorsing the idea that the patient’s life is worthless. Health care providers must reassure the patient of continued care and commitment. The patient should be educated about alternative, less controversial options, such as symptom management and withdrawing any unwanted treatments and the reality of euthanasia and/or physician-assisted suicide, because the patient may have misconceptions about their effectiveness as well as the legal implications of the choice. Depression, hopelessness, and other symptoms of psychological distress as well as physical suffering and economic burdens are likely factors motivating the request, and such factors should be assessed and treated aggressively. After these interventions and clarification of options, most patients proceed with another approach, declining life-sustaining interventions, possibly including refusal of nutrition and hydration.
CARE DURING THE LAST HOURS
Most laypersons have limited experiences with the actual dying process and death. They frequently do not know what to expect of the final hours and afterward. The family and other caregivers must be prepared, especially if the plan is for the patient to die at home.
Patients in the last days of life typically experience extreme weakness and fatigue and become bedbound; this can lead to pressure sores. The issue of turning patients who are near the end of life, however, must be balanced against the potential discomfort that movement may cause. Patients stop eating and drinking with drying of mucosal membranes and dysphagia. Careful attention to oral swabbing, lubricants for lips, and use of artificial tears can provide a form of care to substitute for attempts at feeding the patient. With loss of the gag reflex and dysphagia, patients may also experience accumulation of oral secretions, producing noises during respiration sometimes called “the death rattle.” Scopolamine can reduce the secretions. Patients also experience changes in respiration with periods of apnea or Cheyne-Stokes breathing. Decreased intravascular volume and cardiac output cause tachycardia, hypotension, peripheral coolness, and livedo reticularis (skin mottling). Patients can have urinary and, less frequently, fecal incontinence. Changes in consciousness and neurologic function generally lead to two different paths to death (Fig. 10-2).
FIGURE 10-2 Common and uncommon clinical courses in the last days of terminally ill patients. (Adapted from FD Ferris et al: Module 4: Palliative care, in Comprehensive Guide for the Care of Persons with HIV Disease. Toronto: Mt. Sinai Hospital and Casey Hospice, 1995, http://www.cpsonline.info/content/resources/hivmodule/module4complete.pdf.)
Each of these terminal changes can cause patients and families distress, requiring reassurance and targeted interventions (Table 10-9). Informing families that these changes might occur and providing them with an information sheet can help preempt problems and minimize distress. Understanding that patients stop eating because they are dying, not dying because they have stopped eating, can reduce family and caregiver anxiety. Similarly, informing the family and caregivers that the “death rattle” may occur and that it is not indicative of suffocation, choking, or pain can reduce their worry from the breathing sounds.
MANAGING CHANGES IN THE PATIENT’S CONDITION DURING THE FINAL DAYS AND HOURS |
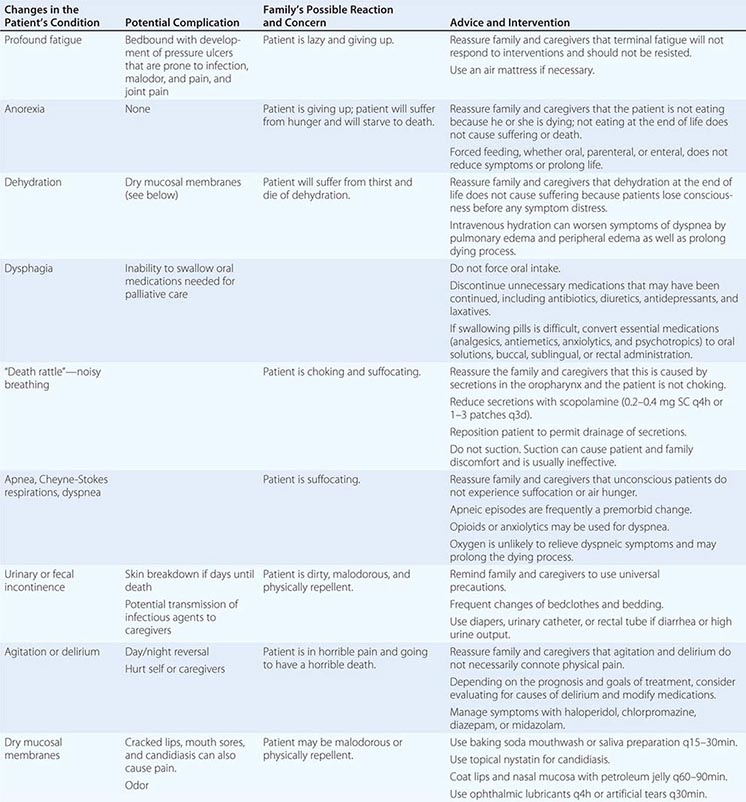
Families and caregivers may also feel guilty about stopping treatments, fearing that they are “killing” the patient. This may lead to demands for interventions, such as feeding tubes, that may be ineffective. In such cases, the physician should remind the family and caregivers about the inevitability of events and the palliative goals. Interventions may prolong the dying process and cause discomfort. Physicians also should emphasize that withholding treatments is both legal and ethical and that the family members are not the cause of the patient’s death. This reassurance may have to be provided multiple times.
Hearing and touch are said to be the last senses to stop functioning. Whether this is the case or not, families and caregivers can be encouraged to communicate with the dying patient. Encouraging them to talk directly to the patient, even if he or she is unconscious, and hold the patient’s hand or demonstrate affection in other ways can be an effective way to channel their urge “to do something” for the patient.
When the plan is for the patient to die at home, the physician must inform the family and caregivers how to determine that the patient has died. The cardinal signs are cessation of cardiac function and respiration; the pupils become fixed; the body becomes cool; muscles relax; and incontinence may occur. Remind the family and caregivers that the eyes may remain open even after the patient has died because the retroorbital fat pad may be depleted, permitting the orbit to fall posteriorly, which makes it difficult for the eyelids to cover the eyeball.
The physician should establish a plan for who the family or caregivers will contact when the patient is dying or has died. Without a plan, they may panic and call 911, unleashing a cascade of unwanted events, from arrival of emergency personnel and resuscitation to hospital admission. The family and caregivers should be instructed to contact the hospice (if one is involved), the covering physician, or the on-call member of the palliative care team. They should also be told that the medical examiner need not be called unless the state requires it for all deaths. Unless foul play is suspected, the health care team need not contact the medical examiner either.
Just after the patient dies, even the best-prepared family may experience shock and loss and be emotionally distraught. They need time to assimilate the event and be comforted. Health care providers are likely to find it meaningful to write a bereavement card or letter to the family. The purpose is to communicate about the patient, perhaps emphasizing the patient’s virtues and the honor it was to care for the patient, and to express concern for the family’s hardship. Some physicians attend the funerals of their patients. Although this is beyond any medical obligation, the presence of the physician can be a source of support to the grieving family and provides an opportunity for closure for the physician.
Death of a spouse is a strong predictor of poor health, and even mortality, for the surviving spouse. It may be important to alert the spouse’s physician about the death so that he or she is aware of symptoms that might require professional attention.
PALLIATIVE CARE SERVICES: HOW AND WHERE
Determining the best approach to providing palliative care to patients will depend on patient preferences, the availability of caregivers and specialized services in close proximity, institutional resources, and reimbursement. Hospice is a leading, but not the only, model of palliative care services. In the United States, a plurality—41.5%—of hospice care is provided in residential homes. In 2012, just over 17% of hospice care was provided in nursing homes. In the United States, Medicare pays for hospice services under Part A, the hospital insurance part of reimbursement. Two physicians must certify that the patient has a prognosis of ≤6 months if the disease runs its usual course. Prognoses are probabilistic by their nature; patients are not required to die within 6 months but rather to have a condition from which half the individuals with it would not be alive within 6 months. Patients sign a hospice enrollment form that states their intent to forgo curative services related to their terminal illness, but they can still receive medical services for other comorbid conditions. Patients also can withdraw enrollment and reenroll later; the hospice Medicare benefit can be revoked later to secure traditional Medicare benefits. Payments to the hospice are per diem (or capitated), not fee-for-service. Payments are intended to cover physician services for the medical direction of the care team; regular home care visits by registered nurses and licensed practical nurses; home health aid and homemaker services; chaplain services; social work services; bereavement counseling; and medical equipment, supplies, and medications. No specific therapy is excluded, and the goal is for each therapy to be considered for its symptomatic (as opposed to disease-modifying) effect. Additional clinical care, including services of the primary physician, is covered by Medicare Part B even while the hospice Medicare benefit is in place. The health reform legislation signed into law in March 2010—the Affordable Care Act—directs the Secretary of Health and Human Services to gather data on Medicare hospice reimbursement with the goal of reforming payment rates to account for resource use over an entire episode of care. The legislation also requires additional evaluations and reviews of eligibility for hospice care by hospice physicians or nurses. Finally, the legislation establishes a demonstration project for concurrent hospice care in Medicare, which would test and evaluate allowing patients to remain eligible for regular Medicare during hospice care.
By 2012, the mean length of enrollment in a hospice was around 71.8 days, with the median being 18.7 days. Such short stays create barriers to establishing high-quality palliative services in patients’ homes and also place financial strains on hospice providers because the initial assessments are resource intensive. Physicians should initiate early referrals to the hospice to allow more time for patients to receive palliative care.
Hospice care has been the main method in the United States for securing palliative services for terminally ill patients. However, efforts are being made to ensure continuity of palliative care across settings and through time. Palliative care services are becoming available as consultative services and more rarely as palliative care units in hospitals, in day care and other outpatient settings, and in nursing homes. Palliative care consultations for nonhospice patients can be billed as for other consultations under Medicare Part B, the physician reimbursement part. Many believe palliative care should be offered to patients regardless of their prognosis. A patient, his or her family, and physicians should not have to make a “curative versus palliative care” decision because it is rarely possible to make such a decisive switch to embracing mortality.
FUTURE DIRECTIONS
OUTCOME MEASURES
Care near the end of life cannot be measured by most of the available validated outcome measures because palliative care does not consider death a bad outcome. Similarly, the family and patients receiving end-of-life care may not desire the elements elicited in current quality-of-life measurements. Symptom control, enhanced family relationships, and quality of bereavement are difficult to measure and are rarely the primary focus of carefully developed or widely used outcome measures. Nevertheless, outcomes are as important in end-of-life care as in any other field of medical care. Specific end-of-life care instruments are being developed both for assessment, such as The Brief Hospice Inventory and NEST (needs near the end of life screening tool), and for outcome measures, such as the Palliative Care Outcomes Scale, as well as for prognosis, such as the Palliative Prognostic Index. The field of end-of-life care is entering an era of evidence-based practice and continuous improvement through clinical trials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree