45
CHAPTER OUTLINE
Opioids include substances that are derived directly from the opium poppy (such as morphine and codeine), the semisynthetic opioids (such as heroin), and the purely synthetic opioids (such as methadone and fentanyl).
These compounds share several pharmacologic effects, including sedation, respiratory depression, and analgesia, and common clinical features of intoxication and withdrawal. This chapter reviews the clinical features of opioid intoxication and withdrawal.
Although all drugs in the class are associated with clinical withdrawal syndromes, those most commonly encountered in clinical practice include heroin, methadone, morphine, oxycodone, codeine, hydrocodone, and meperidine (1).
OPIOID INTOXICATION AND OVERDOSE
Clinical Picture
The prevalence of opioid use in the United States continues to increase. According to the results of the National Survey on Drug Use and Health, among individuals 12 years of age or older, self-reported lifetime heroin use has increased from 1.2% in 2000 to 1.6% in 2010. Similarly, the lifetime nonmedical use of prescription opioids among individuals 12 years of age and older has increased from 8.6% in 2000 to 13.7% in 2010 (1). Opioid intoxication and overdose may present in a variety of settings. Although mild to moderate intoxication (as evidenced by euphoria or sedation) usually is not life threatening, severe intoxication or overdose is a medical emergency that causes many preventable deaths and thus requires immediate attention (2). In a retrospective analysis of consecutive cases of presumed opioid overdose in patients initially managed by emergency medical services personnel in an urban setting, 16% were either dead or in full cardiopulmonary arrest at the time of the initial emergency medical service evaluation (3). As the prevalence of opioid use has increased in the United States, the incidence of opioid overdose has increased as well. For example, one population-based study performed in King County, WA, demonstrated a 134% increase in the number of opioid overdose deaths between 1990 and 1999 (4). Additionally, for patients prescribed opioids for chronic, noncancer pain, overdose risk increases in a dose-dependent fashion. Of 9,940 patients receiving prescription opioids in a managed care organization from 1997 to 2005, 51 overdoses were noted, 6 of which were fatal. Compared to patients receiving 1 to 20 mg/d of opioid equivalents, those receiving 50 to 99 mg/d had a 3.7-fold increased risk of overdose, and those receiving 100 or more mg per day had an 8.9-fold increase in overdose risk (5). Accidental overdose may occur in a variety of settings. A recent epidemic of more than 1,000 accidental overdoses occurred in several US cities where heroin was mixed with more potent opioids (e.g., fentanyl) or experienced heroin users began to use more potent opioids such as fentanyl. This epidemic, occurred in 2005 in Chicago, Detroit, Philadelphia, and rural areas along the East coast, was unlike prior epidemics of its kind because it had far-reaching geographic effects (6).
Increases in opioid overdose deaths have been seen outside the United States as well (7). Despite these increases, opioid overdose can be treated successfully, if patients present in a timely manner and general principles of overdose management (as well as specific therapies for opioid overdose) are employed. In a retrospective analysis in Finland, the survival-to-hospital discharge rate of cardiopulmonary arrest after heroin overdose (16%) was found to be similar to that of other poisonings (11%) (8).
Nonfatal opioid overdose is an additional cause of significant morbidity, and the true prevalence may not be well understood because many nonfatal overdoses are not brought to medical attention (9). The prevalence of nonfatal overdose ranged from 10% to 69% as reported in recent literature (9–11). The factors associated with nonfatal opioid overdose include injection as the route of administration, sporadic heroin use, needing help with injection, prior overdose, and polydrug use.
The pharmacologic actions responsible for opioid intoxication and overdose involve a specific set of opioid receptors, particularly those in the central nervous system (CNS) (12,13). These opioid receptors include the mu, kappa, and delta types, which also interact with endogenous substances, including the endorphins (14). Of primary concern in the management of overdose are interactions with mu receptors in the CNS, which can lead to sedation and respiratory depression. The mechanism of respiratory depression with opioids presumably is direct suppression of respiratory centers in the brain stem and medulla (12).
The level of tolerance to opioids can have a significant effect on an individual’s risk of opioid overdose. In addition, tolerance to respiratory depression may be slower than tolerance to euphoric effects, thus explaining why overdose occurs so often, even among “experienced” opioid users (15). Detoxified patients or those who have experienced intentional or unintentional abstinence from opioids for any reason (e.g., incarceration) may be particularly susceptible to death from heroin overdose (16). Nonfatal overdose is also common among recently detoxified patients—occurring in 27% of a cohort of 201 opioid-dependent patients followed for 2 years after detoxification. Among this group, prior overdose attempts and depressive symptoms were shown to be risk factors for nonfatal overdose (17). Although injecting opioids may be the route of administration associated with the highest risk of overdose, increasingly popular noninjection routes are associated with significant risk as well (18).
Diagnosis
As with most clinical challenges, evaluation of opioid intoxication begins with the collection of patient data through a detailed history and physical examination (Table 45-1). An important issue in the patient with moderate to severe respiratory depression is the immediate institution of pharmacologic and supportive therapies to ameliorate morbidity and prevent mortality.
TABLE 45-1 DIAGNOSIS OF OPIOID OVERDOSE

When available, historical information can be obtained concerning opioid use (including the specific drug, amount, and time of last use); either directly from the patient or from friends and family members, this information can supplement available hospital records. In addition to opioid, it is important to ask about use of other drugs or alcohol because of the likelihood use of more than one drug (19,20). Identification of polydrug use has important implications for patient management; for example, identification of the frequent co-occurrence of opioid and benzodiazepine overdose may indicate the need for additional therapy directed at reversing the benzodiazepine component of the overdose with flumazenil (21). This also is true in cases of suspected opioid overdose in children who are at high risk of co-occurring opioid and benzodiazepine toxicity and who thus may require management of both on presentation for medical care (22). Polydrug overdose often accounts for significant morbidity and mortality. In one retrospective study, more than half of all drug overdose deaths were a result of polydrug overdose with opiates, alcohol, and cocaine (23).
Physical examination of the opioid-intoxicated patient may find CNS and respiratory depression, as well as miosis and direct evidence of drug use, such as needle tracks or soft tissue infection. The heroin overdose syndrome, described as a triad of altered mental status, depressed respiration, and miotic pupils, has a sensitivity of 92% and a specificity of 76% for the diagnosis of heroin overdose (2). Additional evidence supports the use of clinical characteristics in the diagnosis of heroin overdose. In a study of 730 patients in Los Angeles receiving naloxone for suspected heroin overdose, the presence of one of the three clinical signs— respirations less than 12 per minute, presence of pinpoint pupils, and circumstantial evidence of opioid use—had a sensitivity of 92% and a specificity of 76%, whereas the sensitivity of naloxone response was 88%, and specificity was 86% (24). The laboratory can also provide important supportive information in the evaluation of opioid intoxication.
It is important to consider the differential diagnosis in patients presenting with symptoms of opioid intoxication. Other possibilities of depressed mental status include hypoglycemia, acidemia or other fluid and electrolyte disorders, or complications from end-stage liver disease. Additionally, acute mental status changes from HIV-related opportunistic infections may mimic those of opioid intoxication (25). Finally, intoxication from other substances should be considered. Therefore, toxicology screening should be performed immediately in emergency settings (26). Urine toxicology is preferred, because urine contains higher concentrations of drugs and their metabolites than serum. Results usually are qualitative, indicating only the presence or absence of specific substances. Even when the results of toxicologic screening are not available until after acute management has been initiated, drug screening may support the diagnosis of drug intoxication and also may reveal the presence of other drugs not suspected on initial evaluation. Benzodiazepine misuse is common among patients with opioid dependence, and some benzodiazepines (such as clonazepam) may not be readily detectable by standard urine techniques. Alternative approaches involving the examination of serum may be useful in documenting benzodiazepine abuse (27).
Kellerman et al. (28) examined the effects of drug screening on suspected overdose in a study of 405 adult patients who presented to an emergency department. Although initial clinical management did not change significantly on the basis of the toxicology results, implications for treatment beyond the acute event were noted. Poor follow-up of drug screening also was demonstrated in a study of alcohol intoxication in patients injured in motor vehicle crashes. In that study, none of 47 patients who had alcohol levels between 200 and 500 mg/dL were referred for a substance abuse follow-up visit (29). Thus, toxicology screening is useful not only for acute management but also for planning care after discharge from the acute setting (30). Referral to alcohol and drug treatment programs from the emergency department may be an effective mechanism to link opioid-dependent patients, who otherwise may not interface with the medical system, with available treatment programs (31).
Opioid use and overdose also may be complicated by the effects of substances employed to “cut” drugs purchased on the street. Along with inert substances present to add bulk, active substances—including dextromethorphan, lidocaine, and scopolamine—may be present. One report of overdoses that contained significant amounts of scopolamine documented the potential clinical importance of this problem (32). Additionally, unusual complications may present as a result of contamination of drugs of abuse. For instance, recent case reports of patients presenting with cutaneous necrosis, purpura, and neutropenia have been linked to levamisole (an antihelminthic, immunomodulatory, and antineoplastic medication)-contaminated cocaine (33).
Although the classic “triad” of respiratory depression, coma, and pinpoint pupils usually alerts clinicians to the possibility of opioid overdose, atypical presentations may cause some initial confusion. In a study of 43 hospitalized patients who received naloxone for a clinically suspected narcotic overdose, only two overdose patients had the classic triad, suggesting that a high index of suspicion should be maintained in certain patients who may have atypical presentations (34).
Management
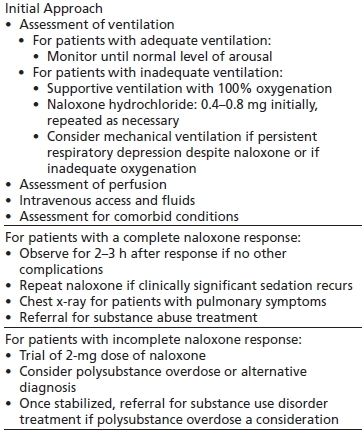
In a case of suspected opioid intoxication, general supportive management must be instituted simultaneously with the specific antidote, naloxone (Table 45-2) (2). Adult basic life support and adult advanced cardiac life support need to be available (35). The physician needs to assure that an adequate airway is established and that respiratory and cardiac function are appropriately assessed and managed. Adequate intravenous access is essential so that fluids and pharmacologic agents can be administered as needed. Finally, frequent monitoring of vital signs and cardiorespiratory status is required until it is clearly established that the opioid and any other intoxicating substances have been cleared from the patient’s system. Additionally, the clinician must consider the half-life of the ingested substance as multiple doses of naloxone or a naloxone drip may need to be instituted in the case of ingesting of a long-acting opioid.
TABLE 45-2 MANAGEMENT OF OPIOID OVERDOSE
In the course of managing patients with suspected opioid overdose, clinicians need to be aware of the co-occurrence of acute medical problems and the exacerbation of chronic medical problems often seen in this population (25,36). For example, prolonged hypoxia in overdose survivors can result in rhabdomyolysis and myocardial infarction (37). Other issues, such as acute infections, trauma, and chronic liver disease (including chronic hepatitis C virus [HCV]), may have major implications for management of the overdose patient (36).
Pharmacologic Therapies
When a patient presents to an emergency department with miosis and respiratory depression, pharmacologic therapy for opioid intoxication should be instituted immediately (2). However, if the patient is breathing without assistance, specific pharmacotherapy should be withheld and the patient monitored (2). Naloxone hydrochloride, a pure opioid antagonist, can effectively reverse the CNS effects of opioid intoxication and overdose. An initial intravenous dose of 0.4 to 0.8 mg will quickly reverse neurologic and cardiorespiratory depression. The onset of action of intravenously administered naloxone, as manifested by antagonism of opioid overdose, is approximately 2 minutes. Although intravenous naloxone should work more rapidly than subcutaneous naloxone, one study demonstrated that the subcutaneous route may be just as effective for managing patients before they arrive in the emergency department; additionally, the slower absorption time of the subcutaneous route may compensate for the delay in establishing adequate intravenous access (38).
Overdose with opioids that are more potent (such as fentanyl) or longer acting (such as methadone) may require higher doses of naloxone given over longer periods of time, as by ongoing naloxone infusion (39). In patients who do not respond to multiple doses of naloxone, alternative causes of the failure to respond must be considered. Along with the need to monitor patients for continued naloxone requirements, another important consideration to anticipate in administering naloxone is the possibility of initiating a significant withdrawal syndrome.
Follow-Up Care
Pharmacologic management of acute opioid overdose is relatively straightforward compared to the challenge of engaging patients with opioid use disorders into medical care and addiction treatment once the overdose event has resolved. In one study of 924 injection drug users in Baltimore, MD, 368 (40%) reported ever having an overdose. Twenty-six percent of the patients with an overdose sought drug treatment within 30 days after the event; the most common reason for seeking treatment was noted to be speaking with someone about treatment options at the time of the overdose. Multiple “missed opportunities” were noted: 87% of overdose patients treated by emergency medical services, 74% of overdose patients treated in the emergency room, and 57% of overdose patients hospitalized denied receiving drug treatment information from the medical staff (40). Despite these and similar findings, clinicians who manage overdose patients should establish the need for ongoing addiction treatment as the major goal of patient management while caring for overdose-related complications.
For medical personnel, two common questions that arise when patients with opioid overdose are seen in the emergency department are which patients can be discharged and when they can be discharged. Clearly, patients with major acute medical or psychiatric comorbidities, including suicidal ideation, should be hospitalized for further treatment. In the absence of these issues, resolution of the symptoms of intoxication and establishment of follow-up referrals for addiction, medical, and psychiatric care are necessary before a patient can be discharged safely. In a study of 573 emergency department patients, a group of investigators developed a clinical prediction rule to identify patients with opioid overdose who could be safely discharged 1 hour after naloxone administration. The authors reported that patients who can be safely discharged are those who can mobilize as usual, have oxygen saturation on room air of greater than 92%, have a respiratory rate greater than 10 breaths/min and less than 20 breaths/min, have a temperature of greater than 35.0°C and less than 37.5°C, have a heart rate greater than 50 beats/min and less than 100 beats/min, and have a Glasgow Coma Scale score of 15. Such patients are at lower risk of adverse events (41).
Recent evidence suggests that naloxone also may have a role in the prevention and treatment of opioid overdose when used in the community by drug users themselves. This concept is based on the fact that most users of illicit opiates have witnessed overdoses and many have witnessed overdose-related deaths (42). A 2005 systematic review noted that although the literature regarding take-home naloxone remains descriptive, there is some evidence to suggest its efficacy in reducing heroin overdose mortality (43). In a British study of 239 opioid-dependent patients, the authors found that patient training and provision of naloxone resulted in improved knowledge of overdose recognition and use of naloxone on 10 occasions in a 3-month period (44). The approach may warrant further research, possibly including controlled clinical trials (45,46).
Other public health approaches aimed at reducing opioid overdose and overdose mortality have been reported in the literature. These include development of supervised injecting facilities (46) and use of diacetylmorphine (i.e., heroin) to treat heroin dependence (47). Although these approaches have been reported as efficacious, their widespread dissemination remains to be seen.
OPIOID WITHDRAWAL
The opioid abstinence syndrome is characterized by two phases (48,49). In the initial phase, opioid-dependent patients experience acute withdrawal. This is followed by the more chronic signs of a protracted abstinence syndrome. Current pharmacotherapeutic strategies are based on this duality.
Acute Withdrawal
In the initial opioid withdrawal phase, the patient typically experiences a range of symptoms for various lengths of time. Such symptoms include gastrointestinal distress (such as diarrhea and vomiting), thermoregulation disturbances, insomnia, muscle and joint pain, and marked anxiety and dysphoria. Although these symptoms generally include no life-threatening complications (unlike alcohol withdrawal syndrome), the acute withdrawal syndrome causes marked discomfort, often prompting continuation of opioid use even in the absence of any opioid-associated euphoria.
Chronic Dependence and Protracted Abstinence
In patients with a chronic history of opioid dependence presenting with acute withdrawal, detoxification is only the beginning of treatment. Himmelsbach (48), reporting on 21 prisoners addicted to morphine, observed that “physical recovery requires not less than 6 months of total abstinence.” Factors he measured included temperature, sleep, respiration, weight, basal metabolic rate, blood pressure, and hematocrit. The times required for return to baseline ranged from 1 week to about 6 months. Martin and Jasinski (49) reported in a subsequent study that this phase persisted for 6 months or more after withdrawal and that it was associated with “altered physiologic function.” They found decreased blood pressure, decreased heart rate and body temperature, miosis, and a decreased sensitivity of the respiratory center to carbon dioxide, beginning about 6 weeks after withdrawal and persisting for 26 or more weeks. They also found increased sedimentation rates (which persisted for months) and electroencephalogram changes.
Martin and Jasinski also postulated a relationship between the protracted abstinence syndrome and relapse. Based on similar observations, Dole (50) concluded that “human addicts almost always return to use narcotics” after hospital detoxification. In his article, he reviewed the relative importance of metabolic and conditioned factors in relapse and concluded that the underlying drive is metabolic, arguing that “psychological factors are only triggers for relapse.”
The concept of protracted abstinence has been controversial (51), but remains a useful model for scientific hypothesis testing and development of new therapeutic approaches (52). Accordingly, Dole recommended methadone maintenance treatment, even though “it does establish physical dependence.” Because, as Dole pointed out, methadone continues physical dependence, protracted abstinence may continue to be a problem whenever detoxification from opioids is undertaken. However, methadone treatment, when prescribed at appropriate doses, provides a “narcotic blockade,” which blocks the euphoric effect of exogenous opioids and stabilizes psychosocial functioning (53–55).
In addition to biologic considerations, psychosocial concomitants of opioid dependence also necessitate longer, more specialized adjunct treatments for these additional problems.
Clinical Picture
Withdrawal from opioids results in a specific constellation of symptoms. Although some opioid withdrawal symptoms overlap withdrawal from sedative–hypnotics, opioid withdrawal generally is considered less likely to produce severe morbidity or mortality. Clinical phenomena associated with opioid withdrawal include neurophysiologic rebound in the organ systems on which opioids have their primary actions (56). Thus, the generalized CNS suppression that occurs with opioid use is replaced by CNS hyperactivity.
The severity of opioid withdrawal varies with the specific opioid used and the dose and duration of drug use. In addition, route of administration appears to be important as well. Data from one study suggest that injection drug use is associated with significantly higher withdrawal symptom scores than was inhaled opioid use for comparable heroin doses (57). The time to onset of opioid withdrawal symptoms depends on the half-life of the drug being used. For example, withdrawal may begin 4 to 6 hours after the last use of heroin, but up to 36 hours after the last use of methadone (56).
Neuropharmacologic studies of opioid withdrawal have supported the clinical picture of CNS noradrenergic hyperactivity (56,58). Therapies to alter the course of opioid withdrawal (such as clonidine) are designed to decrease this hyperactivity, which occurs primarily at the locus caeruleus (59,60). Evidence for the role of noradrenergic hyperactivity in opioid withdrawal has been provided by studies showing elevated norepinephrine metabolite levels (61).
Diagnosis
Opioid withdrawal involves a constellation of clinical manifestations. Several clinical tools are available to measure the severity of opioid withdrawal. One such tool is the Clinical Opiate Withdrawal Scale (Table 45-3) (62). Other validated scales can also be employed for assessment. These include the 10-item Short Opioid Withdrawal Scale, which takes less than a minute to administer (63); the 16-item Subjective Opioid Withdrawal Scale, and the 13-item Objective Opioid Withdrawal Scale (64). Early findings may include abnormalities in vital signs, including tachycardia and hypertension. Bothersome CNS system symptoms include restlessness, irritability, and insomnia. Opioid craving also occurs in proportion to the severity of physiologic withdrawal symptoms. Pupillary dilation can be marked. A variety of cutaneous and mucocutaneous symptoms (including lacrimation, rhinorrhea, and piloerection—also known as “gooseflesh”) can occur as well. Patients frequently report yawning and sneezing. Gastrointestinal symptoms, which initially may be mild (anorexia), can progress in moderate to severe withdrawal to include nausea, vomiting, and diarrhea. This combination of uncomfortable symptomatology and intense craving frequently leads to relapse to drug use (51).
TABLE 45-3 CLINICAL OPIATE WITHDRAWAL SCALE

Adapted from “Flowsheet for measuring symptoms during buprenorphine induction.” Available at: www.naabt.org. Accessed November 1, 2007.
As with the onset of withdrawal, the duration also varies with the half-life of the drug used and the duration of drug use. For example, the meperidine abstinence syndrome may peak within 8 to 12 hours and last only 4 to 5 days (56), whereas heroin withdrawal symptoms generally peak within 36 to 72 hours and may last for 7 to 14 days (50).
A protracted abstinence syndrome has been described, in which a variety of symptoms may last beyond the typical acute withdrawal period (65). Findings in prolonged and protracted abstinence may include mild abnormalities in vital signs and continued craving (66). Despite the extensive literature on protracted withdrawal, a universal definition and diagnostic criteria are lacking, making diagnosis difficult in individual patients (51).
Management
As in the management of opioid intoxication and overdose, management of opioid withdrawal involves a combination of general supportive measures and specific pharmacologic therapies. It is very important for the physician to do a thorough evaluation to rule out other medical problems that may be complicating the opioid withdrawal. The choice of pharmacotherapy used to treat withdrawal may be influenced by the presence and severity of a patient’s underlying medical comorbidities (30).
In addition to assessment of general health problems, it is important to obtain objective information to help guide the management of patients undergoing opioid withdrawal. Thus, a physical examination should be performed to detect specific findings consistent with withdrawal to establish the diagnosis.
General supportive measures for managing withdrawal include providing a safe environment and adequate nutrition, as well as reassuring patients that their symptoms will be taken seriously. The decision as to whether to perform opioid detoxification on an outpatient or inpatient basis depends on the presence of comorbid medical and psychiatric problems, the availability of social supports (such as family members to provide monitoring and transportation), and the presence of polydrug use. Access to methods of detoxification also may affect this decision; for example, methadone detoxification has been restricted by federal legislation to inpatient settings or specialized licensed outpatient drug treatment programs (67); more recent federal initiatives allow some opioid-based treatments to be used under less restricted circumstances (68,69).
In the course of managing opioid withdrawal, clinicians also need to be able to address medical problems seen in this population (25,36). Issues such as acute bacterial infections, HIV, and HCV-related problems may complicate withdrawal presentation and management. For instance, some studies suggest diminished expression of endogenous interferon-α and enhanced HCV viral replication in patients both using and withdrawing from opioids suggesting that opioid use and withdrawal favor HCV persistence in hepatocytes (70), whereas other studies suggest that intravenous drug use increases cytokine response in patients coinfected with HIV and HCV (71).
Pharmacologic Therapies for Opioid Withdrawal
Several pharmacologic therapies have been developed to assist patients through a safer, more comfortable opioid withdrawal. These therapies involve the use of opioid agonists (such as methadone); alpha-2 adrenergic agonists (such as clonidine); an opioid antagonist (such as naltrexone or naloxone) in combination with clonidine, with sedation, or with general anesthesia; or partial opioid agonist (buprenorphine) (72).
Full Opioid Agonists
Slow Methadone Detoxification
It is important to distinguish between withdrawal from short-acting opioids such as heroin (plasma half-life of morphine, the main metabolite: 3 to 4 hours) and long-acting opioids such as methadone (plasma half-life: 13 to 47 hours). For short-acting opioids, the natural course of withdrawal generally is relatively brief, but more intense and associated with a higher degree of discomfort than with equivalent doses of long-acting opioids. However, there is considerable individual variation, so that strong early withdrawal symptoms from methadone are possible, as are delayed severe heroin withdrawal symptoms.
One treatment strategy employing this general principle is to stabilize patients dependent on heroin with methadone and then gradually decrease the methadone dose over months rather than days. Initially, methadone may be given in 5- to 10-mg increments, p.r.n., as the physical signs of abstinence begin to appear (71), up to a total of 30 to 40 mg over the first 24 hours. This treatment strategy can only be employed by facilities licensed to prescribe methadone for the treatment of opioid dependence.
The protocol for slow methadone detoxification is similar to the strategy used for withdrawal from methadone maintenance treatment. After a stabilizing dose has been reached, methadone is tapered by 20% a day for inpatients, leading to a 1- to 2-week procedure. Alternatively, the dose is tapered by 5% per day for outpatients, in a gradual cessation phase lasting as long as 6 months (73). Senay et al. (74) studied the effects of rapid (reductions of 10% of initial dose per week) and gradual (3% per week) outpatient cessation under double-blind conditions. They found that the 10% weekly decrements were associated with higher dropout rates, increased illicit opioid use, and elevated levels of subjective distress. The authors recommended a dose-tapering rate of about 3% per week from methadone maintenance. On such a regimen, successful detoxification can be achieved by as many as 80% of inpatients and 40% of outpatients, when success is measured in terms of completion of detoxification and a withdrawal-free naloxone challenge test. The longer duration of the procedure and the greater discomfort make the outpatient detoxification with methadone especially vulnerable to patient dropout and continuing illicit opioid use. One study showed that even when coupled with enhanced psychosocial counseling, patients enrolled in 6-month methadone detoxification programs demonstrated greater illicit opioid use and greater drug-related HIV risk behaviors than patients enrolled in methadone maintenance (75).
Other Opioid Agonists
Detoxification from methadone maintenance treatment can be especially challenging. Recent work has focused on protocols to treat symptoms associated with withdrawal from methadone. Data from observational studies and randomized clinical trials suggest that slow release morphine is as effective as methadone in terms of opioid withdrawal, opioid craving, or self-reported symptoms (76,77).
Alpha-2 Adrenergic Agents
Clonidine Detoxification
Gold et al. (59) reported amelioration of opioid withdrawal symptoms by use of clonidine and postulated that both morphine and clonidine blocked activation of the locus caeruleus, a major noradrenergic nucleus that shows increased activity during opioid withdrawal. Although opioids exert their effect through opiate receptors, clonidine activates alpha-2 adrenergic receptors. Consequently, clonidine does not possess the potential for physical dependence and abuse seen with opioids.
In a subsequent outpatient study (78), clonidine was reported to “reduce or eliminate most of the commonly reported withdrawal symptoms,” including lacrimation, rhinorrhea, restlessness, muscle pain, joint pain, and gastrointestinal symptoms. However, symptoms such as lethargy and insomnia persisted. Sedation and dizziness from ortho-static hypotension were reported as the most significant side effects of clonidine. Though clonidine has been shown to be useful to decrease symptoms associated with opioid withdrawal, its use for this purpose is considered off-label, and because it is not an opioid agonist, many symptoms of opioid withdrawal remain untreated though they can be ameliorated by a number of other adjunctive treatments (i.e., nonsteroidal anti-inflammatory drugs, bismuth subsalicylate, pharmacotherapy for insomnia, dicyclomine).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


