43
CHAPTER OUTLINE
Management of alcohol intoxication and withdrawal is one of the clinical issues most frequently encountered by specialists in addiction medicine. Effective approaches, with a strong scientific basis, have been developed to reduce the incidence of serious complications.
ALCOHOL INTOXICATION
Clinical Picture
As blood alcohol concentration rises, so too does the clinical effect on the individual (Table 43-1) (1). At a blood alcohol concentration between 20 mg% and 99 mg%, loss of muscular coordination begins, and changes in mood, personality, and behavior occur. As the blood alcohol level rises to the range of 100 mg% to 199 mg%, neurologic impairment occurs, accompanied by prolonged reaction time, ataxia, incoordination, and mental impairment. At a blood alcohol level of 200 mg% to 299 mg%, obvious intoxication is present, except in those persons with marked tolerance. Nausea and vomiting, as well as marked ataxia, may occur. In very young and/or naïve drinkers, levels of 300 mg% may be associated with coma and death, especially when levels are reached quickly (e.g., “chugging” drinks).
TABLE 43-1 CLINICAL EFFECTS OF ALCOHOL
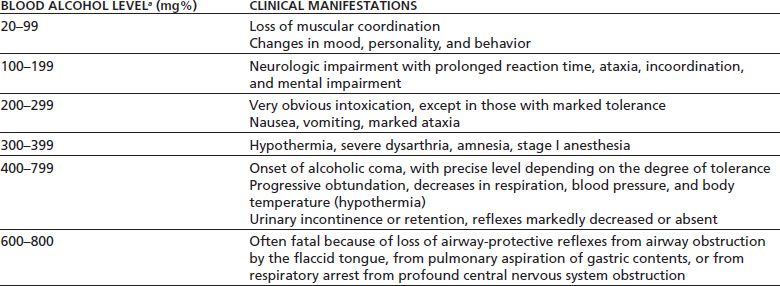
aLevels of 200–300 mg%, particularly when reached quickly (“chugging”), may result in coma, aspiration, and death in nontolerant individuals, particularly adolescents and young adults. In addition, presence of other depressant drugs, even in therapeutic doses (benzodiazepines, sedative–hypnotics, opioids), may result in respiratory depression, coma, and death at lower levels of alcohol.
As the level rises to 300 mg% to 399 mg%, hypothermia may occur, along with severe dysarthria and amnesia, with stage I anesthesia. At blood alcohol levels between 400 mg% and 799 mg%, the onset of alcoholic coma occurs. The precise level at which this occurs depends on tolerance; some persons experience coma at levels of 300 mg%, whereas others do not experience it until the level approaches 600 mg%, depending largely on tolerance as well as the rapidity of reaching the peak blood alcohol concentration. Blood levels of alcohol between 600 mg% and 800 mg% are commonly fatal. Progressive obtundation develops, accompanied by decreases in respiration, blood pressure, and body temperature. The patient may develop urinary incontinence or retention, while reflexes are markedly decreased or absent. Death may occur from the loss of airway-protective reflexes (with subsequent airway obstruction by the flaccid tongue), from pulmonary aspiration of gastric contents, or from respiratory arrest arising from profound central nervous system depression.
Management
The medical management of alcohol intoxication and overdose is supportive. The primary goal of management of alcohol intoxication is to prevent harm to the patient, from severe respiratory depression, and to protect the airway against aspiration. Even with very high blood alcohol levels, survival is probable as long as the respiratory and cardiovascular systems can be supported. Attention must be paid to the potential presence of nonbeverage alcohol (methyl alcohol, isopropyl alcohol, or ethylene glycol) as well as coingestion of other toxins (benzodiazepines, tricyclic antidepressants, etc.), since these intoxications may present a similar clinical picture, but require very different management.
Medical treatment is supportive in the alcohol-intoxicated patient. As with all patients with impaired consciousness, intravenous glucose should be given if rapid testing of blood glucose is not immediately available, as well as intravenous thiamine. These are of particular importance in alcohol intoxication as ethanol can impair gluconeogenesis, with an increased risk of hypoglycemia, and alcohol use disorder places the individual at an increased risk of thiamine deficiency. Whenever feasible, intravenous thiamine should be given before glucose, particularly if given in large amounts or high concentrations.
It also is important to assess whether the patient has ingested other drugs in addition to alcohol, because these drugs may further suppress the central nervous system and alter the approach to treatment. Alcohol is rapidly absorbed into the bloodstream, so induction of emesis or gastric lavage is not indicated unless a substantial ingestion has occurred within the preceding 30 to 60 minutes or when other drug ingestion is suspected. Induced emesis may be useful at the scene (e.g., with children at home) if it can be given within a few minutes of exposure. However, syrup of ipecac is no longer routinely recommended, because of its potential toxicity if induction of emesis is unsuccessful. Mechanical induction of emesis (i.e., stimulating the gag reflex with fingers or instruments) has been discouraged because of the possibility of trauma to the upper airway. Similarly, gastric lavage is indicated only if the patient presents in the emergency department soon after ingestion. Activated charcoal does not efficiently absorb ethanol, but may be given if other toxins have been ingested.
More than 90% of alcohol is oxidized in the liver, and, at the levels seen clinically, the rate of oxidation follows zero-order kinetics; alcohol metabolism is independent of time and concentration of the drug. Elimination thus occurs at a fixed rate, with the level falling at a rate of about 20 mg/dL/h. In extreme cases of alcohol intoxication, hemodialysis (or peritoneal dialysis) can be used, because it efficiently removes alcohol, but it is needed only rarely because supportive care usually is sufficient. Hemoperfusion and forced diuresis are not effective. At present, there is no known agent that is effective as an alcohol antagonist, reversing the effects of alcohol in the same manner that naloxone reverses opioid intoxication. Importantly, benzodiazepine antagonists such as flumazenil do not block or reverse alcohol intoxication.
The acutely intoxicated patient may exhibit some agitation as part of the intoxication syndrome. This is best managed nonpharmacologically. Support and reassurance can go a long way in dealing with agitation in an acutely intoxicated patient. On rare occasions, if pharmacologic intervention is needed to manage a mildly or moderately intoxicated individual’s behavior in a medical setting, intramuscular administration of a rapid-onset, short-acting benzodiazepine, alone or in combination with a neuroleptic agent such as haloperidol, can be useful. Caution must be exercised in regard to a potential synergistic response between the alcohol already in the patient’s system and an exogenously administered sedative–hypnotic, so this approach should be used only as a last resort and not in individuals with high blood alcohol levels. If such an approach is used, low doses (i.e., haloperidol 1 mg with lorazepam 1 mg) are recommended. Higher benzodiazepine doses may result in respiratory depression, vomiting, and aspiration, as well as the potential for “paradoxical” increased agitation by increasing intoxication.
HANGOVER
Hangover is a constellation of unpleasant physical and mental symptoms that occur after heavy alcohol intake. Headache, malaise, diarrhea, nausea, and difficulty concentrating are the most common symptoms, often accompanied by sensitivity to light or sound, sweating, and anxiety. About 75% of individuals who drink to intoxication report experiencing a hangover at least some of the time. The primary alcohol-related morbidity in light to moderate drinkers is hangover. Because light to moderate drinkers make up most of the workforce, the greatest cost incurred by alcohol in the workplace is the decreased productivity as a result of hangover-induced absenteeism or poor job performance. In addition to the personal discomfort, hangover increases the risk for injury and poor job performance. Patients with hangover have diminished visual–spatial skills and dexterity with impairment demonstrated in pilots, automobile drivers, and skiers. There are also adverse effects on managerial skills and tasks (2).
The pathophysiology of hangover is not completely understood. In part, it is believed to be the effect of the intermediate product of ethanol metabolism, acetaldehyde. In addition, congeners, by-products of individual alcohol preparations found primarily in dark liquors such as brandy, whiskey, wine, and tequila, appear to play a role because they increase the frequency and severity of hangover. Clear liquors, such as rum, vodka, and gin, cause hangover less frequently (2,3). Dehydration, electrolyte imbalance, disruption of sleep and other biologic rhythms, increased physical activity while intoxicated, hypoglycemia, and the many hormonal disruptions caused by alcohol may also play contributing roles (3). Patients with hangover have a diffuse slowing on electroencephalography, which may persist up to 16 hours after blood alcohol levels become undetectable (2).
Although many interventions have been tried to alleviate hangover symptoms, to date, none has clearly demonstrated effectiveness in rigorous investigations (4). Conservative management offers the best course of treatment, and symptoms generally resolve over 8 to 24 hours. Attentiveness to the quantity and quality of alcohol consumed can have a significant effect on preventing hangover. Asking patients about their hangover experiences offers an opportunity for education on a common cause of physical, psychiatric, and occupational consequences of drinking alcohol.
ALCOHOL WITHDRAWAL
Clinical Presentation
The relationship of heavy alcohol intake to certain syn dromes has been recognized since ancient times (Hippocrates, circa 400 BC). However, it was not until the 18th century that the clinical manifestations of alcohol withdrawal were clearly delineated. As is evident in the writings of Sutton, the vivid descriptions of severe withdrawal written at that time remain relevant today:
It is preceded by tremors of the hands, restlessness, irregularity of thought, deficiency of memory, anxiety to be company, dreadful nocturnal dreams when the quantity of liquor through the day has been insufficient: much diminution of appetite, especially an aversion to animal food; violent vomiting in the morning and excessive perspiration from trivial causes. Confusion of thought arises to such height that objects are seen of the most hideous forms, and in positions that it is physically impossible they can be so situated; the patients generally sees flies or other insects; or pieces of money which he anxiously desires to possess… (5)
For the most part, clinicians believed that these symptoms were a consequence of alcohol itself. It was not until the second half of the 20th century that their relationship to the cessation of chronic alcohol intake—a relationship taken for granted today—was established. In 1953, Victor and Adams (6) reported their careful observations of 286 consecutive alcohol-dependent patients admitted to an inner city hospital, revealing the consistent relationship of the cessation of alcohol to the emergence of clinical symptoms. Their findings were supported in 1955 by a study by Isbell et al. (7), in which 10 former morphine-addicted individuals were given large quantities of alcohol for 7 to 87 days and then withdrawn abruptly without sedation. Over the next two decades, the concept of an alcohol withdrawal syndrome was firmly established by further animal and human studies, and diagnostic criteria based on empirical observation were developed. Today, manifestations of alcohol withdrawal are generally categorized in clinical practice as including the common alcohol withdrawal syndrome as well as more severe manifestations of hallucinations, seizures, and delirium.
Alcohol Withdrawal Syndrome
The current understanding of the alcohol withdrawal syndrome is reflected in the diagnostic criteria of the Diagnostic and Statistical Manual of the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, fourth and fifth editions (8,9). In those with physiologic dependence on alcohol, the clinical manifestations of alcohol withdrawal begin 6 to 24 hours after the last drink (or after marked reduction in the quantity of alcohol consumed), sometimes arising before the blood alcohol level has returned to zero. Early withdrawal signs and symptoms include anxiety, sleep disturbances, vivid dreams, anorexia, nausea, and headache. Physical signs include tachycardia, elevation of blood pressure, hyperactive reflexes, sweating, and hyperthermia. A tremor, best brought out by extension of the hands or tongue, may appear. This tremor has a rate of six to eight cycles per second and appears on electromyography to be an exaggeration of normal physiologic tremor. The severity of these symptoms varies greatly among individuals, but in a majority, they are mild and transient, passing within 1 to 2 days (10–12).
Hallucinations
In mild alcohol withdrawal, patients may experience perceptual distortions of a visual, auditory, and tactile nature. Lights may seem too bright or sounds too loud and startling. A sensation of “pins and needles” may be experienced. In more severe cases of withdrawal, these misperceptions may develop into frank hallucinations. Visual hallucinations are most common and frequently involve some type of animal life, such as seeing a dog or rodent in the room. Auditory hallucinations may begin as unformed sounds (such as clicks or buzzing) and progress to hearing voices. In contrast to the auditory hallucinations of schizophrenia, which may be of religious or political significance, these voices often are of friends or relatives and frequently are accusatory in nature. Tactile hallucinations may involve a sensation of bugs or insects crawling on the skin. In milder cases of withdrawal, the patient’s sensorium is otherwise clear, and the patient retains insight that the hallucinations are not real. In more severe withdrawal, this insight may be lost. Hallucinosis can occur in the absence of other withdrawal symptoms. They should be distinguished from the hallucinosis that can be part of delirium tremens (DTs) (see below).
Alcohol Withdrawal Seizures
Grand mal seizures are another manifestation of alcohol withdrawal. Withdrawal seizures occurred in 23% of the patients studied by Victor and Adams, in 33% of the patients in Isbell’s study who drank for 48 to 87 days, and in 11% of placebo-treated patients who were enrolled in prospective controlled studies examining the effectiveness of benzodiazepines in symptomatic withdrawal (6,7,12,13). Withdrawal seizures usually begin within 8 to 24 hours after the patient’s last drink and may occur before the blood alcohol level has returned to zero. Most are generalized major motor seizures, occurring singly or in a burst of several seizures over a period of 1 to 6 hours. Although less than 3% of withdrawal seizures evolve into status epilepticus, alcohol withdrawal has been found to be a contributing cause in up to 15% of status epilepticus patients (15). Like hallucinosis, seizures can occur in an absence of other withdrawal symptoms.
Seizures peak 24 hours after the last drink, corresponding to the peak of withdrawal-induced electroencephalogram (EEG) abnormalities, which include increased amplitude, a photomyoclonic response, and spontaneous paroxysmal activity. These EEG abnormalities are transient, in keeping with the brevity of the convulsive attacks. Except for this brief period after withdrawal, the incidence of EEG abnormalities in patients with withdrawal seizures is not greater than in the normal population. The risk of withdrawal seizures appears to be in part genetically determined and is increased in patients with past withdrawal seizures or in those who are undergoing concurrent withdrawal from benzodiazepines or other sedative–hypnotic drugs. The use of benzodiazepines increases the likelihood of status epilepticus when withdrawing from both drugs.
There also is evidence that the risk of seizures increases as an individual undergoes repeated withdrawals (15). This association has been described as a “kindling effect,” which refers to animal studies demonstrating that repeated sub-cortical electrical stimulation is associated with increases in seizure susceptibility (16). Animal studies have supported this kindling hypothesis in alcohol withdrawal, demonstrating that submitting animals to repeated alcohol withdrawal episodes increases their risk of withdrawal seizures. There is emerging evidence that this effect occurs in humans as well (15–21).
Alcohol Withdrawal Delirium
Withdrawal is highly individualized in both severity and duration. For up to 90% of patients, withdrawal does not progress beyond the mild to moderate symptoms described previously, peaking between 24 and 36 hours and gradually subsiding. In other patients, however, manifestations can include delirium. The diagnostic criteria are similar to those of the newly released DSM-5 (9). In the classic cases of withdrawal delirium, the manifestations of withdrawal steadily worsen and progress into a severe life-threatening delirium accompanied by an autonomic storm: hence the term delirium tremens (DTs). DTs generally appear 72 to 96 hours after the last drink. In their classic presentation, DTs are marked by all the signs and symptoms of mild withdrawal but in a much more pronounced form, with the development of marked tachycardia, tremor, diaphoresis, and fever. The patient develops global confusion and disorientation to place and time. The patient may become absorbed in a separate psychic reality, often believing him-or herself to be in a location other than the hospital, and misidentifies staff as personal acquaintances. Hallucinations are frequent, and the patient may have no insight into them. Without this insight, they can be extremely frightening to the patient, who may react in a way that poses a threat to the patients’ or the staff’s safety.
Marked psychomotor activity may develop, with severe agitation in some cases or continuous low-level motor activity in others, so that activities such as efforts to get out of bed can last for hours. Severe disruption of the normal sleep–wake cycle also is common and may be marked by the absence of clear sleep for several days. The duration of the delirium is variable, but averages 2 to 3 days in most studies (22). In some cases, the delirium is relatively brief, lasting only a few hours before the patient regains orientation. In other cases, the patient remains delirious for several days, with reports of periods as long as 50 days before the confusion clears (23). Before the development of effective treatment, mortality in DTs was substantial. With the development of effective therapy, including intensive care, death from DTs is an unusual event (14,23).
Because the clinical syndrome of delirium has become more carefully and broadly studied, and standard diagnostic criteria developed, it is becoming apparent that many cases of delirium during alcohol withdrawal occur without the autonomic storm associated with classically described DTs. Although the terms “alcohol withdrawal delirium” and “DTs” are often used interchangeably (7), many cases of delirium in alcohol withdrawal are mild and transient. In one retrospective chart review of 284 patients undergoing withdrawal, 20 patients were identified as meeting DSM-IV-R criteria for alcohol withdrawal delirium, while only 3 had the syndrome of DTs (25). This is an area needing further investigation, including the role that treatment with sedative–hypnotics or concurrent medical illness may play in the development of mild delirium in alcohol withdrawal.
Alcohol Withdrawal Severity Scales
Because alcohol withdrawal involves a constellation of nonspecific findings, efforts have been made to develop structured withdrawal severity assessment scales to objectively quantify the severity of withdrawal. Several such scales have been published in the literature (26,27). The most extensively studied and best known is the Clinical Institute Withdrawal Assessment—Alcohol, or CIWA, and a shortened version known as the CIWA-A Revised, or Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) (Table 43-2) (27,28). The CIWA-Ar has well-documented reliability, reproducibility, and validity based on comparisons to ratings of withdrawal severity by experienced clinicians. The CIWA-Ar and similar scales require 2 to 5 minutes to complete and have proved useful in a variety of settings, including detoxification units, psychiatric units, medical/surgical wards, and intensive care units. Such scales allow rapid documentation of the patient’s signs and symptoms and provide a simple summary score that facilitates accurate and objective communication among staff. In the case of the CIWA-Ar, a score ≤9 indicates mild withdrawal, a score of 10 to 18 moderate withdrawal, and a score greater than 18 severe withdrawal.
TABLE 43-2 CLINICAL INSTITUTE WITHDRAWAL ASSESSMENT OF ALCOHOL SCALE, REVISED (CIWA-AR)

This assessment for monitoring withdrawal symptoms requires approximately 5 min to administer. The maximum score is 67 (see instrument). Patients scoring less than 10 do not usually need additional medication for withdrawal.
From Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar). Br J Addict 1989;84:1353–1357. (27).
A careful analysis of symptoms recorded using withdrawal scales found that patients segregated into distinct clinical groups. Approximately 20% had no significant withdrawal symptoms (10). Another 20% had only vegetative (physical) signs, such as tremor and sweating, but no psychological symptoms. The largest group, approximately 40%, had both vegetative and mild to moderate psychological symptoms, primarily anxiety. The last group, about 20% of patients, had both vegetative and severe psychological symptoms with disorientation, delirium, or hallucinations. As indicated previously, relatively few patients in alcohol withdrawal experience the adrenergic and clinical manifestations of DTs.
It is extremely important that staff responsible for patient assessment be adequately trained in the utilization of the CIWA-Ar. One common error is to assess the patient who is clinically intoxicated, which may result in a high score because of nonspecific responses (nausea, headache, anxiety), and thus allow treatment of an already intoxicated individual with benzodiazepines, with the potential for increased intoxication, respiratory depression, vomiting and aspiration, and other complications. The responsible clinician (physician, nurse practitioner, physicians’ assistant) should be thoroughly familiar with the instrument and be able to train nurses and other health professionals who will be monitoring the patients. Interobserver variability may occur and lead to inappropriate scoring (high or low) when the observers have not had adequate training in differentiating the differing anchor points given in the scoring instrument.
Professionals using the CIWA-Ar need to understand that it is not a diagnostic instrument for alcohol withdrawal; the clinician must diagnose alcohol withdrawal on the basis of the clinical setting. When the withdrawal syndrome is recognized, the CIWA-Ar can assist in determining its severity and assist in establishing the effects of treatment on its course. Clinicians utilizing such scales must understand that there may also be confounding factors present, which may increase the score (concomitant dehydration, infection, other causes of hyperautonomic signs and symptoms) or decrease it (advanced age, use of beta-blockers or other sympatholytic drugs).
Predictors of Severe Withdrawal
Withdrawal scales can also contribute to appropriate triage of patients as it has been shown that high scores early in the course are predictive of the development of seizures and delirium. Those with low scores on withdrawal scales over the first 24 hours, particularly in those with such scores who have taken no benzodiazepines, have consistently been found to be at little or no risk for severe withdrawal. Other risk factors for severe withdrawal include a history of prior DTs or withdrawal seizures. Marked autonomic hyperactivity, commonly measured as elevated heart rate on admission, elevated blood alcohol level of 100 mg/dL or higher at the time of admission, serum electrolyte abnormalities, and medical comorbidity, particularly infection, are other clinical findings associated with an increased rate of DTs or severe withdrawal. Characteristics that have not been useful in tri-aging patients include the amount of daily intake, duration of heavy drinking, age, and gender. At least one rating system has been developed combining multiple items to predict the severity of withdrawal (11). This and other studies were able to identify patients at low risk for severe withdrawal with high reliability. The clinical utility of this rating system has not been established and likely requires further study.
Pathophysiology
Goldstein and Goldstein (29) proposed in 1961 that dependency develops as a cell or organism makes homeostatic adjustments to compensate for the primary effect of a drug. The primary effect of alcohol on the central nervous system is as a depressant. With chronic exposure, there are compensatory adjustments to this chronic depressant effect, with down-regulation of inhibitory systems and up-regulation of excitatory systems. With abrupt abstinence from alcohol, these relative deficiencies in inhibitory influences and relative excesses in excitatory influences are suddenly unmasked, leading to the appearance of withdrawal phenomena. The withdrawal symptoms last until the body readjusts to the absence of the alcohol and establishes a new equilibrium.
Two neurotransmitter systems appear to play a central role in the development of alcohol withdrawal syndrome. Alcohol exerts its effects in part by directly or indirectly enhancing the effect of GABA, a major inhibitory neurotransmitter. GABA mediates typical sedative–hypnotic effects such as sedation, muscle relaxation, and a raised seizure threshold. Chronic alcohol intake leads to an adaptive suppression of GABA activity. A sudden relative deficiency in GABA neurotransmitter activity is produced with alcohol abstinence and is believed to contribute to the anxiety, increased psychomotor activity, and predisposition to seizures seen in withdrawal. Although alcohol enhances the effect of GABA, it inhibits the sensitivity of the autonomic adrenergic systems, with a resulting up-regulation with chronic alcohol intake. The discontinuation of alcohol leads to rebound over activity of the brain and peripheral noradrenergic systems. Increased sympathetic autonomic activity contributes to such acute manifestations as tachycardia, hypertension, tremor, diaphoresis, and anxiety (30).
A second neurotransmitter, norepinephrine, also seems to be important in alcohol withdrawal presentations. Norepinephrine’s metabolites are elevated in plasma, urine, and cerebral spinal fluid during withdrawal; levels of metabolites correlate significantly with the sympathetic nervous system signs of withdrawal (31). Research has identified a large number of other neural effects of chronic alcohol intake, including effects on serotonergic systems, neuronal calcium channels, glutamate receptors, cyclic AMP systems, and the hypothalamic–pituitary–adrenal neuroendocrine axis, and these too may play a role in the pathophysiology of withdrawal (29). In addition, more recent studies suggest that the glutamatergic system and NMDA dysregulation play a role in the development of CNS excitation as well, particularly seizures and delirium (32).
Genetics
The role of genetics in alcohol withdrawal is a topic of active investigation. In animal models, the development of selectively bred strains demonstrates that severity of withdrawal and risk of seizures are strongly influenced by genotype. Investigations in humans have focused on genes regulating neurotransmitter systems. Several studies have found an association of the A9 allele, which affects central dopamine functions, with severity of alcohol withdrawal, alcohol withdrawal seizures, and DTs (33–35). To date, no relationship with genes involved in the serotonin, GABAergic, or endorphinergic systems have been found (36). Although these findings are not of immediate clinical use, genetic studies may shed light on basic pathophysiology and, at some point, assist in identifying high-risk individuals who may benefit from tailored therapy.
Role of Alcohol Withdrawal in Diagnostic Classification
When DSM-III-R moved to a broad definition of dependence, it removed any requirement for physiologic components, tolerance and withdrawal, for a diagnosis of alcohol dependence. This was done without intensive study of what effect would occur if neither was required for a diagnosis. DSM-IV did add a request to subtype dependence into groups with and without physiologic components. Intervening studies have shown that approximately 60% of individuals meeting DSM-IV criteria for alcohol dependence report withdrawal symptoms, another 35% report tolerance without withdrawal symptoms, and only 5% report neither. Of the seven DSM-IV dependence items, withdrawal symptoms have been most strongly associated with an increased number of alcohol-related problems, higher number of drinks per occasion, and future difficulties (35). Alternative approaches to DSM-IV have been proposed that reinstitute withdrawal as a required feature, with evidence that such approaches offer better validity and better discrimination between the dependence and abuse classifications (37). The role of withdrawal in the diagnostic taxonomy of alcohol use disorders continues to be investigated and debated.
While the DSM-5 eliminates the abuse/dependence dichotomy, and instead uses Substance Use Disorder-Alcohol, based on the presence of 11 criteria (the same as the current criteria except with recurrent legal difficulties being replaced by a craving criterion) (38), and with severity indicators for absent (0 to 1 criterion), mild (2 to 3 criteria), moderate (4 to 5 criteria), and severe (6 or greater criteria) (9), definitions and criteria for alcohol withdrawal syndromes are not significantly different from those in the DSM-IV-TR (9).
MANAGEMENT OF ALCOHOL WITHDRAWAL SYNDROMES
General Principles
The primary goals of the treatment of alcohol withdrawal syndromes are to first assure clinical stability of the patient and secondarily encourage ongoing treatment (e.g., rehabilitation) of a patient’s alcohol use disorder. The first step in managing the patient with alcohol withdrawal is to perform an assessment for the presence of medical and psychiatric problems. Chronic alcohol intake is associated with the development of many acute and chronic medical problems. The clinician needs to determine whether there are acute conditions that require hospital treatment or chronic conditions that may alter the approach to management of withdrawal because they could be exacerbated significantly by the development of withdrawal or its treatment. Pertinent laboratory tests generally include complete blood count, electrolytes, magnesium, calcium, phosphate, liver enzymes, urine drug screen, pregnancy test (when appropriate), and breath or blood alcohol level.
Others, depending on suspected co-occurring conditions, may include skin test for tuberculosis, chest x-ray, electrocardiogram, and tests for viral hepatitis, other infections, or sexually transmitted diseases. General management also involves maintaining adequate fluid balance, correction of electrolyte deficiencies, and attendance to the patient’s nutritional needs. Patients in early withdrawal often are overhydrated so that aggressive hydration usually is not necessary unless there have been significant fluid losses from vomiting or diarrhea. Supportive care and reassurance from health care personnel are important elements of comfortable detoxification and help to facilitate continuing treatment. Supportive nonpharmacologic care is an important and useful element in the management of all patients undergoing withdrawal. Simple interventions such as reassurance, reality orientation, monitoring of signs and symptoms of withdrawal, and general nursing care are effective.
There has been interest in the possible value of complementary and alternative medicine for alcohol withdrawal. Controlled trials of acupuncture have not demonstrated effectiveness (39,40) whereas massage therapy did reduce alcohol withdrawal scores (41). It is important to note that all these supportive measures do not prevent the development of major complications such as seizures and are not adequate by themselves to manage the patient with or at elevated risk for severe withdrawal or delirium, in which case pharmacologic intervention is required.
Pharmacologic Management of Uncomplicated Withdrawal Syndrome
The medical literature on the pharmacologic management of alcohol withdrawal has been comprehensively reviewed as part of the American Society of Addiction Medicine’s (ASAM’s) evidence-based clinical practice guidelines efforts (14,23). This review of the evidence indicated that the cornerstone of pharmacologic management of withdrawal is the use of benzodiazepines, a conclusion supported by other more recent systematic reviews (41,42).
Benzodiazepines
Benzodiazepines are pharmacologically cross-tolerant with alcohol and have the similar effect of enhancing the effect of GABA-induced sedation. A specific benzodiazepine receptor site has been identified on the GABA receptor complex. It is believed that the provision of benzodiazepines alleviates the acute deficiency of GABA neurotransmitter activity that occurs with sudden cessation of alcohol intake. Well-designed studies consistently have shown that benzodiazepines are more effective than placebo in reducing the signs and symptoms of withdrawal.
Meta-analyses of prospective placebo-controlled trials of patients admitted with symptomatic withdrawal have shown a highly significant reduction in seizures, with a risk reduction of 7.7 seizures per 100 patients treated (14), as well as in delirium, with a risk reduction of 4.9 cases of delirium per 100 patients treated (23). Trials comparing different benzodiazepines indicate that all are similarly efficacious in reducing signs and symptoms of withdrawal. However, longer-acting agents such as diazepam and chlordiazepoxide may be more effective in preventing seizures. Longer-acting agents also may contribute to an overall smoother withdrawal course, with a reduction in breakthrough or rebound symptoms. On the other hand, pharmacologic data and clinical experience suggest that longer-acting agents can pose a risk of excess sedation in some patients, including elderly persons and patients with significant liver disease (hepatic synthetic dysfunction), and in patients with chronic pulmonary disease, where prolonged respiratory depression may occur. In such patients, shorter-acting agents such as lorazepam or oxazepam may be preferable.
Another consideration in the choice of benzodiazepine is the rapidity of onset. Certain agents with rapid onset of action (such as diazepam, alprazolam, and lorazepam) demonstrate greater abuse potential than do agents with a slower onset of action (such as chlordiazepoxide and oxazepam). This consideration may be of relevance in an outpatient setting or for patients with a history of benzodiazepine or other substance abuse. However, when rapid control of symptoms is needed, medications with faster onset offer an advantage. A final consideration in the choice of benzodiazepine is cost, as these agents vary considerably in price. Given the evidence of equal efficacy, if a particular agent is available to a practitioner or program at a lower cost, cost is a legitimate factor to consider.
The pharmacokinetics of different benzodiazepines should be taken into consideration dependent upon the clinical circumstances, including the age and health of the patient, potential drug–drug interactions, and the stage and severity of withdrawal. Younger, healthier patients generally tolerate longer-acting drugs, which may produce a smoother course. Shorter-acting agents may be better tolerated in older, sicker patients, particularly those with hepatic insufficiency and/or pulmonary disease. However, if patients are assessed for signs of oversedation prior to each dose, and at peak levels, of benzodiazepine received, oversedation can be avoided, even if longer-acting agents are employed. Drug latency may also be an issue when patients present with moderate to severe withdrawal, since several commonly used drugs have longer periods between oral ingestion and peak levels, such as oxazepam (which takes 1.5 to 2 hours to peak) or chlordiazepoxide (1 to 2 hours), while diazepam may reach peak levels in 20 to 30 minutes. Since oxazepam is relatively short acting, the long latency and the rapid excretion may produce a “choppy” course; in those with hepatic disease, lorazepam may be an equally effective and safe option, with both more rapid absorption and a longer period of clinical activity. Attention to tapering doses can be important when shorter-acting agents are used as dependence to them can develop rapidly and withdrawal from them can lead to symptoms including seizures. While dependence upon benzodiazepines given therapeutically is a legitimate concern, it rarely occurs unless the benzodiazepine has been prescribed for 10 to 14 days, although the author has rarely seen shorter courses leading to withdrawal.
Studies have indicated that nonbenzodiazepine sedative– hypnotics also are effective in reducing the signs and symptoms of withdrawal, but nonbenzodiazepine agents have not been as extensively studied, and the size of studies with them is not adequate to draw conclusions as to their degree of effectiveness in reducing seizures and delirium. Benzodiazepines have a greater margin of safety, with a lower risk of respiratory depression, as well as overall lower abuse potential than do the nonbenzodiazepine agents. Phenobarbital, a long-acting barbiturate, still is used by some programs, as it is long acting, has well-documented anticonvulsant activity, is inexpensive, and has low abuse liability. However, as with all barbiturates, oversedation is commonly associated with both depressed consciousness and potential respiratory depression. Barbiturates have not been shown to reduce seizures or delirium in alcohol withdrawal. It is critically important to assess every patient for signs and symptoms of oversedation (sustained nystagmus, dysarthria, dysmetria, ataxia, mood lability, and depressed level of consciousness) prior to receiving additional doses and to withhold additional doses if such signs or symptoms are present. Chlormethiazole, an agent combing benzodiazepine and anticonvulsant effects, while widely used elsewhere, is unavailable in the United States.
Determining the Dosing Schedule
In the majority of studies examining the effectiveness of various medications for withdrawal, the medications were given in fixed amounts at scheduled times (such as chlordiazepoxide 50 mg every 6 hours) and were given for periods of 5 to 7 days. However, it has been shown that many patients can go through withdrawal with only minor symptoms even though they receive little or no medication (13,14). An alternative to giving medication on a fixed schedule is known as symptom-triggered therapy. In this approach, the patient is monitored through use of a structured assessment scale and given medication only when symptoms cross a threshold of severity. Well-designed studies have demonstrated that this approach is as effective as fixed-dose therapy, but leads to the administration of significantly less medication and a significantly shorter duration of treatment (12,13).
Symptom-triggered therapy also facilitates the delivery of large amounts of medication quickly to patients who evidence rapidly escalating withdrawal and thus reduces the risk of undertreatment that may arise with the use of fixed doses. For programs specializing in the management of addiction, use of a symptom-triggered approach with the utilization of a severity scale offers significant advantages. However, there may be situations in which the provision of fixed doses remains appropriate. For example, in patients admitted to general medical or surgical wards, the nursing staff may not have the training or experience to implement the regular use of scales to monitor patients. In certain patients, such as those with severe coronary artery disease, the clinician may wish to prevent the development of even minor symptoms of withdrawal. Finally, because a history of past withdrawal seizures is a risk factor for seizures during a withdrawal episode, and because withdrawal seizures usually occur early in the course of withdrawal, some practitioners administer fixed doses (or at least one single dose regardless of symptoms) to patients with a history of withdrawal seizures or who are otherwise at higher risk for symptomatic withdrawal such as those with acute medical, surgical, or psychiatric illnesses. Whenever fixed doses are given, it is very important that allowances be made to provide additional medication if the fixed dose should prove inadequate to control symptoms.
Loading dose therapy, which involves monitoring the patient with CIWA-Ar scores until they reach a predetermined set point (generally 8 to 15) and then giving diazepam (10 to 20 mg), clorazepate (3.75 to 7.5 mg), or chlordiazepoxide (50 to 100 mg) on an hourly basis, evaluating the patient prior to each dose until symptoms diminish or signs of oversedation develop—sustained nystagmus, ataxia, dysarthria, mood lability, or oversedation itself (somnolence not responsive to normal-level voice) (43). The patient is then monitored for 2 to 3 hours without further medication. The average patient received 3 to 5 doses before reaching the end point, and 80% of patients were adequately treated with a single episode of loading, while an additional 10% required a second loading dose regimen within the first 24 hours. One-tenth of patients could not be successfully treated with this approach, presumably because of up-regulation of p450 hepatic cytochrome enzymes in these patients, so that usual longer-acting benzodiazepines in effect were shorter acting in these patients.
Treatment should allow for a degree of individualization so that patients can receive large amounts of medication rapidly if needed. In all cases, medications should be administered by a route that has been shown to have reliable absorption. Therefore, the benzodiazepines should be administered orally or, when necessary, intravenously. An exception is lorazepam, which has good intramuscular and sublingual absorption. In the past, intramuscular administration was commonly used. However, for most agents, intramuscular absorption is extremely variable, leading to problems when rapid control of symptoms is necessary and also with delayed appearance of oversedation when large amounts are administered. Examples of some treatment regimens consistent with current recommendations are shown in Table 43-3.
TABLE 43-3 EXAMPLES OF SPECIFIC PHARMACOLOGIC TREATMENT REGIMENS

Adapted from Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA 1997;278:144–151; and Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium. An evidence-based practice guideline.Arch Intern Med 2004;164:1405–1412.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


