Malignant Disorders of White Blood Cells
Marie L. Kotter and Jacquelyn L. Banasik
Key Questions
• Why are malignant disorders of white blood cells commonly associated with bone marrow depression?
• How is Hodgkin disease clinically and histologically differentiated from other types of lymphoma?
• What is the purpose and process of staging procedures for lymphomas?
• What clinical and laboratory findings would suggest a diagnosis of plasma cell myeloma?
![]()
http://evolve.elsevier.com/Copstead/
Leukemia, lymphoma, and plasma cell myeloma (multiple myeloma) are common neoplastic disorders of the bone marrow and lymphoid tissues. Depending on the location and specific types of white blood cells involved, these malignancies can be further divided into a number of specific subtypes. Leukemias can be conceptualized as circulating tumors that are disseminated from the beginning of the disease process and primarily involve the blood and bone marrow. Lymphoma tends to localize in lymph tissues but is often disseminated to other sites at the time of diagnosis. Plasma cell myeloma is a malignant transformation of B cell plasma cells and has a predilection to form localized tumors in bony structures.
Malignancies of the blood-forming tissues and lymphatic structures often present with nonspecific symptoms. Malaise, weakness, unexplained fever, night sweats, and recurrent infections should raise suspicion of malignancy. Enlarged, nontender lymph nodes (lymphadenopathy) are a common finding in lymphoma and some leukemias. Often, white blood cell malignancies are found by chance during routine assessment of the complete blood cell count (CBC). A very high total white blood cell count or the presence of abnormal cell types should precipitate an assessment for hematologic cell malignancy. In general, earlier detection of malignancy is associated with a better prognosis for cure.
Classification of Hematologic Neoplasms
Various classification schemes have been used to group hematologic neoplasms, with clinicians favoring schemes that use clinical findings and pathologists preferring morphologic criteria. With the advent of technologies to identify specific genetic alterations and molecular characteristics of neoplastic cells, the traditional classification systems have become less useful. However, many clinicians and organizations, such as the American Cancer Society, continue to use traditional groupings to collect statistics and to provide information to the public. The approach used in this chapter incorporates the most recent World Health Organization (WHO) classifications for hematologic neoplasms and also includes common clinical terminology. A major force behind the adoption of the WHO classification is the recognition that lymphoid leukemias and lymphomas are not separate disorders but represent different stages of the same biological disease. Thus, the major categories of the WHO system are based on the cell type of the neoplasm, rather than its location in the body.1 Neoplasms involving cells of the myeloid lineage (Box 11-1) are separated from those of the lymphoid lineage (Box 11-2). The myeloid lineage includes red blood cells, platelets, monocytes, and granulocytes; the lymphoid lineage includes B cells, T cells, and natural killer (NK) cells (Figure 11-1). There are four major categories of myeloid neoplasms: myeloproliferative diseases; myelodysplastic/proliferative diseases; myelodysplastic syndromes; and acute myeloid leukemia (AML). There are three major categories of lymphoid neoplasms: B-cell neoplasm; T-cell and NK-cell neoplasm; and Hodgkin disease. The term non-Hodgkin lymphoma is still in clinical usage and refers to lymphomas of B-cell, T-cell, and NK-cell origin. Non-Hodgkin lymphoma includes such a large and diverse group of malignancies that it has little relevance to prognosis or treatment. The WHO classification does not use this term. Other classification systems in current use include the FAB (French-American-British) system for subtypes of myeloid leukemia (Table 11-1). There are many etiologic, pathogenic, and treatment similarities among the hematologic malignancies, and these are addressed in a general way first, followed by sections concentrating on specific diseases.
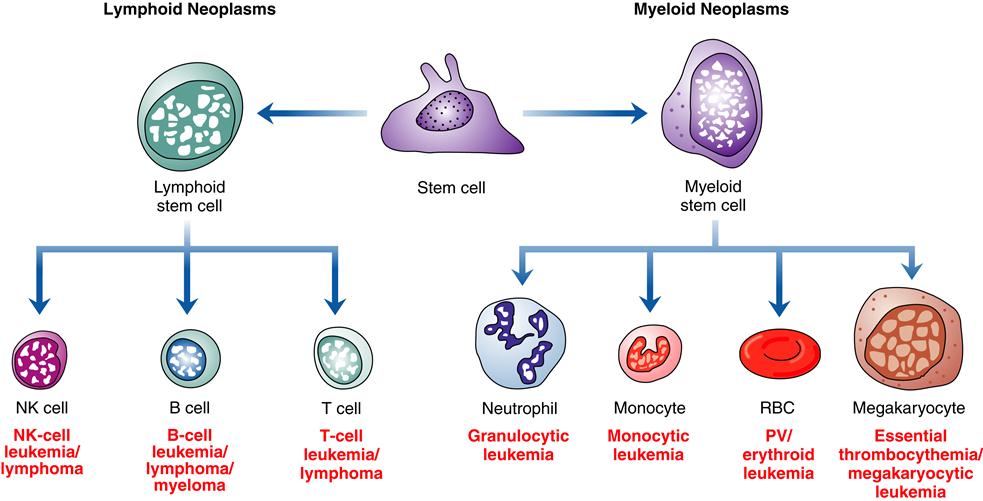
PV, Polycythemia vera.
TABLE 11-1
FAB CLASSIFICATION OF ACUTE MYELOBLASTIC (MYELOCYTIC) LEUKEMIAS
| CLASS | MORPHOLOGY | COMMENTS |
| M0: Minimally differentiated AML | Blasts lack definitive cytologic and cytochemical markers of myeloblasts but express myeloid lineage antigens | 2% to 3% of AML |
| M1: AML without differentiation | Very immature myeloblasts predominate; few granules or Auer rods | 20% of AML; Ph chromosome present in 10% to 15% of cases, worsens prognosis |
| M2: AML with differentiation | Myeloblasts and promyelocytes predominate; Auer rods commonly present | 30% of AML; presence of t(8;21) translocation associated with good prognosis |
| M3: Acute promyelocytic leukemia | Hypergranular promyelocytes, often with many Auer rods per cell; may have reniform or bilobed nuclei | 5% to 10% of AML; disseminated intravascular coagulation common; presence of t(15;17) translocation is characteristic; responds to retinoic acid therapy |
| M4: Acute myelomonocytic leukemia | Myelocytic and monocytic differentiation evident; myeloid elements resemble M2; peripheral monocytosis | 20% to 30% of AML; presence of inv16 or del16q associated with better prognosis |
| M5: Acute monocytic leukemia | Monoblasts (peroxidase negative, esterase positive) and promonocytes predominate | 10% of AML; usually in children and young adults; gum infiltration common; associated with abnormalities of chromosome 11q23 |
| M6: Acute erythroleukemia | Bizarre, multinucleated, megaloblastoid erythroblasts predominate; myeloblasts also present | 5% of AML; high blood counts and organ infiltration are rare; affected persons are of advanced age |
| M7: Acute megakaryocytic leukemia | Blasts of megakaryocytic lineage predominate; react with antiplatelet antibodies; myelofibrosis or increased bone marrow reticulin |
AML, Acute myeloblastic (myelocytic) leukemia; FAB, French-American-British; Ph, Philadelphia.
From Kumar V, Cotran RS, Robbins SL, editors: Basic pathology, ed 7, Philadelphia, 2003, Saunders, p 437.
Etiology of Myeloid and Lymphoid Neoplasms
As in other malignant processes, the exact cause of hematologic neoplasms is unknown. The basic mechanism of malignant transformation involves mutation of cells, which disrupts growth control and differentiation pathways. These processes are thought to be similar to those described for solid tumors (see Chapter 7).
Viruses have long been suspected as mutagenic agents in some neoplasms, particularly retroviruses and herpesviruses. Close associations have been found between a small number of viruses and particular malignancies. For example, human T-cell leukemia virus (HTLV-1) is linked to the development of adult T cell lymphoma/leukemia and human immunodeficiency virus (HIV) is linked to B-cell lymphomas. Epstein-Barr virus (EBV) has been implicated in both Hodgkin disease and Burkitt lymphoma.2 Effective immune surveillance is thought to keep proliferation in check and prevent progression in immunocompetent individuals.
Radiation exposure is an important etiologic factor for leukemia and lymphoma. Because of the relatively high turnover of hematologic cells, they are more susceptible to radiation-induced damage than most other cell types. An acute whole-body dose of radiation like that which occurs with nuclear explosions is known to increase the risk of leukemia. In Japanese survivors of the atomic bomb, the estimated lifetime risk of leukemia is 0.85%, sixfold higher than the norm.3 There are substantial uncertainties about the risk of low-level, long-term exposure to radiation. The average annual exposure from usual sources including cosmic rays and medical procedures is very low and estimated to account for less than 5% of leukemia cases.
Despite intensive scrutiny only a small number of chemicals have been shown unequivocally to increase the risk of hematologic malignancies. Benzene has been implicated in numerous studies as has cigarette smoking. Other suggested carcinogens have failed to be confirmed, including exposure to hair dye, alcohol, and marijuana.4 On the other hand, a study from the Children’s Cancer Group found a link between high maternal intake of products high in bioflavonoids (beans, fresh vegetables, and fruit) and an increased incidence of infant leukemia.5 These bioflavonoids were enzyme inhibitors (topoisomerase II inhibitors) that caused DNA cleavage and chromosome translocations. Many of the antineoplastic drugs used to treat cancers, especially the alkylating agents, are significant factors in the development of posttreatment hematologic neoplasia. Any drugs that suppress the bone marrow or immune function are also believed to predispose to the emergence of malignancies.
A number of disease conditions have been linked to the development of leukemia, although the mechanisms are unclear. A reduction or alteration in normal hematopoiesis, as occurs in such disorders as Fanconi anemia and aplastic anemia (see Chapter 13), is associated with a higher incidence of leukemia. A higher risk also has been noted in some genetic diseases, including Down syndrome and Klinefelter syndrome (see Chapter 6).
General Principles of Management
Diagnosis of Hematologic Neoplasms
Manifestations of hematologic neoplasms vary somewhat, depending upon the cell type involved. Common manifestations are shown in Box 11-3. Clinical symptoms are related to bone marrow suppression and organ dysfunction secondary to leukemic infiltration. Bone marrow suppression results in varying degrees of leukopenia, anemia, and thrombocytopenia. These three deficiencies cause the most common clinical manifestations and may prompt the patient to seek care.
Anemia, with a hematocrit level of 25% to 30% or a hemoglobin level of 8 to 10 g/dl, may manifest with pallor, fatigue, malaise, shortness of breath, and decreased activity tolerance. The severity of symptoms is determined by the rate of red blood cell decrease as well as the absolute deficiency. Chronically low hemoglobin and hematocrit values may be better tolerated than a drastic drop in these measurements. Depending on symptoms, transfusion may be indicated when the hematocrit level falls below 30%.
Thrombocytopenia, with a platelet count less than 20,000 cells/μL, can manifest as petechiae, easy bruising, bleeding gums, occult hematuria, or retinal hemorrhages. Spontaneous intracranial bleeding can occur and may be fatal. In general, the risk of bleeding increases proportionately to the fall in platelet count. Platelet transfusion may be given when the risk of bleeding is high.
Insufficient numbers of functional leukocytes leaves the patient at high risk for development of infection and the complete blood count is routinely monitored. Neutropenia is an absolute neutrophil count less than 500 cells/μL, and an affected patient requires protective isolation (neutropenic precautions) to prevent infection. Infections may be caused by bacterial, viral, fungal, or protozoal organisms. Often, the microorganisms are of the opportunistic variety. That is, they are part of the patient’s own flora, which normally do not cause disease unless the host’s immune system becomes incompetent. It is very difficult to protect patients from their own flora, and infection is the most common cause of death in the immunocompromised leukemic patient. The presence of infection is suspected if fever develops. Infections are managed aggressively with antibiotic agents to prevent development of life-threatening sepsis.
Infiltrative manifestations include lymphadenopathy, joint swelling and pain, weight loss, anorexia, hepatomegaly, and splenomegaly. Sternal tenderness is frequently present in chronic myeloid leukemia (CML). Gingival hyperplasia occurs in acute myeloid leukemia (AML). Meningeal involvement is frequently encountered in children with acute lymphoid leukemia (ALL). Central nervous system (CNS) infiltration can occur with any type of leukemia and may be difficult to manage because of the poor ability of chemotherapeutic agents to cross the blood-brain barrier. CNS involvement can present with increased intracranial pressure, seizures, or changes in mental ability. Increased intracranial pressure should be suspected in the leukemia patient who complains of nausea, vomiting, headache, and visual changes.
A key aspect of diagnosis is the evaluation of a peripheral blood sample. Blood cell number and morphologic evaluation are indicative; however, definitive diagnosis is usually made after bone marrow aspiration or lymph node biopsy. Malignant cells can be subtyped according to genetic and molecular characteristics to better determine prognosis and choice of treatment.6
Principles of Treatment
To make informed treatment decisions, patients and their families need information about the nature and prognosis of their disease as well as about the risks and benefits of various treatment options. Many treatment protocols are experimental, and the outcomes may be uncertain. Sometimes the side effects of treatment as well as its limited efficacy will weigh in favor of palliative care. Treatment decisions are complex and stressful for all concerned. A great deal of support must be available during the diagnostic, treatment, and monitoring phases.
The management of hematologic malignancies relies primarily on the use of combination chemotherapy to eradicate malignant cells and stem cell transplant to rescue and restore bone marrow function. In some cases radiation and tissue-specific drug therapy may be indicated. Unfortunately, the treatment regimen usually causes many serious side effects that must be monitored and treated.
The goal of chemotherapy is to induce long-term remission, that is, the absence of any detectable neoplastic cells in the body. A complete remission (CR) is defined as a return to normal hematopoiesis with normal red blood cell, neutrophil, and platelet counts and no detectable neoplastic cells. For leukemia, the bone marrow must have less than 5% blasts, which are the most immature bone marrow cells, and be maintained for at least 4 weeks.7 CR is not synonymous with cure. Therefore, most treatment protocols include several cycles of chemotherapy to eradicate the undetected cells. The choice of antineoplastic agents varies with the type of neoplasia and the stage of clinical disease. Most chemotherapeutic agents work by disrupting some aspect of DNA synthesis or cell replication and induce apoptosis (cell suicide; see Chapter 4). In general, rapidly dividing cells are more susceptible to apoptosis because they have less time for repair. Neoplasms with genetic defects that impair apoptotic pathways may be more difficult to eradicate and require more intense therapy. Unfortunately, these high doses are toxic to normal stem cells as well and can produce fatal bone marrow failure. Therefore, to effect a cure, high-dose chemotherapy is often followed by bone marrow “rescue” with transplantation of functional stem cells.
Chemotherapy usually includes two or three treatment phases: (1) remission induction phase, (2) postremission or consolidation phase, and (3) remission maintenance phase. The aim of treatment during the remission induction phase is to eliminate all detectable neoplastic cells and achieve a CR. Postremission consolidation therapy begins after CR is attained in an attempt to eliminate the population of undetected cells that may have escaped initial induction phase treatment. Maintenance phase treatment is used in the management of some neoplasms to prolong the remission interval. Intermittent chemotherapy may be continued for 2 to 3 years after initial induction of remission. Drugs that target the neoplastic cells specifically, such as monoclonal antibodies or molecular therapies, are generally less toxic than other agents and may be used for long-term maintenance in patients with residual disease.
In children and adults, the CNS can act as a sanctuary for neoplastic cells in diseases such as acute lymphoblastic and acute myeloblastic leukemias. This makes conventional routes of chemotherapy unsuccessful, because they do not permit drugs to cross the blood-brain barrier efficiently. Chemotherapeutic agents administered into the cerebrospinal fluid (CSF) via lumbar puncture (intrathecal route) can effectively eliminate leukemic cells in the CNS. This therapy carries significant risk for temporary or permanent neurologic damage. A number of different chemotherapeutic agents can be administered safely by the intrathecal route, including methotrexate.8
Prevention and Management of Complications
Maintenance of adequate nutrition in patients with hematologic malignancy is a major challenge. Anorexia, weight loss, nausea, vomiting, and stomatitis are common findings, especially during the treatment phase. Children and adolescents receiving chemotherapy may experience significant growth delay, and measures to maintain protein and caloric intake are necessary. Newer antiemetic agents have been helpful in reducing nausea, vomiting, and anorexia associated with chemotherapy and should be considered in patients experiencing these symptoms.
Infection is the most troublesome of complications for the patient who is immunosuppressed by either disease or treatments. Constant vigilance in prevention, early detection, and rapid management of infections can profoundly affect the outcome of chemotherapy. The length of time that a patient remains neutropenic can be shortened with the use of growth factors to stimulate bone marrow production of granulocytes.
Bone marrow transplantation (BMT) has been an important part of the management of certain leukemias for many years. The intense chemotherapy used to induce remission can lead to bone marrow failure. Stem cells can be reintroduced into the host’s bone marrow by bone marrow transplantation. The transplanted cells are given intravenously; they find their way to the host’s bone marrow, where they establish residence and begin to produce functional white blood cells, red blood cells, and platelets. A close match between donor and host is necessary for a successful transplantation. Otherwise, the transplanted cells can mount an immune attack on the host’s tissues—a life-threatening problem called graft-versus-host disease (see Chapter 10). In past years, bone transplantation was obtained by aspiration from the marrow of a suitable donor and this is still appropriate in some cases. Peripheral stem cell transplantation allows stem cells to be harvested from the circulating bloodstream. This procedure can be used to collect stem cells from the patient’s own blood to be stored and then reinfused after chemotherapy and irradiation. This type of transplant is called autologous, whereas a transplant from a closely matched relative is called allogeneic (Figure 11-2). Use of autologous transplants eliminates the problem of graft-versus-host disease and reduces transplant-related mortality, but the potential for disease recurrence is higher than with allogeneic transplants.
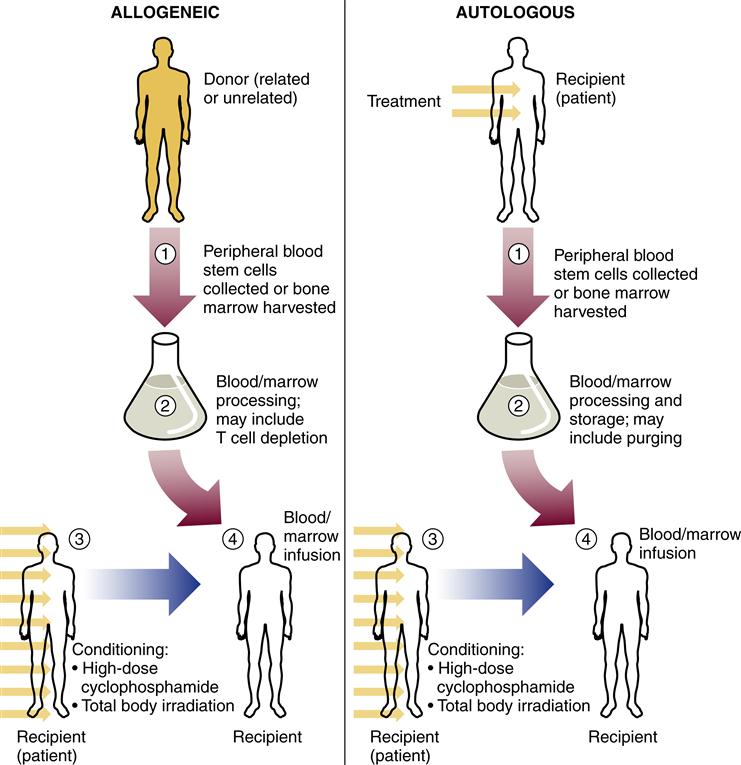
For nonmyeloblative (reduced intensity) allogeneic stem cell transplantation, lower doses of chemotherapy with or without radiotherapy are used. (From Rodak B et al: Hematology: clinical principles and application, ed 4, Philadelphia, 2012, Saunders.)
It has been noted that in AML and CML, transplantation with allogeneic cells is much more successful in curing leukemia than is autologous transplantation. Transplanted cells in the allograft are thought to detect and kill leukemic cells in a process termed graft versus leukemia. Autologous transplants are appropriate in some cases, especially when a matched donor is not available, because they may extend life even though cure is unlikely. Autologous transplants are well tolerated and cause fewer complications than allografts. Methods to purify a patient’s collected peripheral blood by selectively removing neoplastic cells are available to reduce the risk of reintroducing malignant cells during autologous transplantation. Increased availability of stem cell transplants allows patients to undergo more intensive chemotherapy, aimed at cure rather than palliation, based on the knowledge that bone marrow rescue is possible.
Anemia is a common complication of leukemia and chemotherapy. Red blood cell production by the bone marrow is suppressed, but the size and shape of red blood cells present in the blood are normal. This is called normocytic, normochromic anemia. Administration of erythropoietin growth factors can enhance red blood cell production and moderate anemic episodes. However, patients frequently require red blood cell transfusion therapy to maintain adequate red blood cell counts. Patients with frequent or significant bleeding episodes are also predisposed to severe anemia, and efforts to prevent bleeding will help to minimize anemia.
Platelet deficiency (thrombocytopenia) with resultant hemorrhage can be a life-threatening complication of leukemia and chemotherapy. In patients at high risk of bleeding, fresh frozen plasma or pooled platelets may be given to inhibit bleeding. Patients must be protected from trauma and may be placed on activity restrictions.
Pain is a common complication of the diagnostic and treatment protocols used in the cancer patient as well as of the disease process itself. Pain most commonly involves the bones and joints, and is due to pressure caused by infiltration and accumulation of neoplastic cells in the bone marrow. Hemarthrosis (bleeding into joints) can cause acute episodes of joint pain. Chemotherapy may help reduce bone pain, as the number of neoplastic cells is reduced drastically. Patients are subjected to numerous painful procedures during diagnosis, treatment, and monitoring. Frequent collection of blood and bone marrow samples, placement of intravenous access lines for drug administration, and manifestation of unpleasant drug side effects all contribute to the pain experience. Nausea and mouth pain (stomatitis) are frequent complaints during chemotherapy. Pain management with a variety of strategies, including narcotic and nonnarcotic drugs, distraction, and biofeedback, is generally helpful. (See Chapter 47 for a discussion of pain and pain management.)
Epithelial cells, with normally high rates of turnover, are particularly susceptible to damage by radiation and chemotherapy. Sloughing of skin and mucous membranes and hair loss (alopecia) are common. Loss of skin and mucous membrane integrity increases the risk of infection and can contribute significantly to the pain and discomfort of treatment. Abnormalities in growth, development, and fertility are complications of particular concern in children undergoing radiation and chemotherapy.