Chapter 9 Malignant disease
Introduction
The term ‘malignant disease’ encompasses a wide range of illnesses, including common ones such as lung, breast and colorectal cancer (Table 9.1) as well as rare ones, like the acute leukaemias. Malignant disease is widely prevalent and, in the West, almost one-third of the population will develop cancer at some time during their life. It is second only to cardiovascular disease as the cause of death. Although the mortality of cancer is high, many advances have been made, both in terms of treatment and in understanding the biology of the disease at the molecular level.
Table 9.1 Relative 5-year survival estimates based on survival probabilities observed during 2000–2001, by sex and site, England and Wales
| 5-year survival (%) | ||
|---|---|---|
| Men | Women | |
Pancreas | 3 | 2 |
Lung | 6 | 6 |
Oesophagus | 7 | 8 |
Stomach | 12 | 13 |
Brain | 13 | 15 |
Multiple myeloma | 24 | 22 |
Ovary |
| 34 |
Leukaemia | 38 | 36 |
Kidney | 45 | 43 |
Colon | 46 | 45 |
Rectum | 45 | 48 |
Non-Hodgkin’s lymphoma | 51 | 52 |
Prostate | 61 |
|
Larynx | 67 |
|
Bladder | 71 | 61 |
Cervix |
| 68 |
Melanoma | 78 | 90 |
Breast |
| 79 |
Hodgkin’s lymphoma | 84 | 83 |
Testis | 95 |
|
The biology of cancer
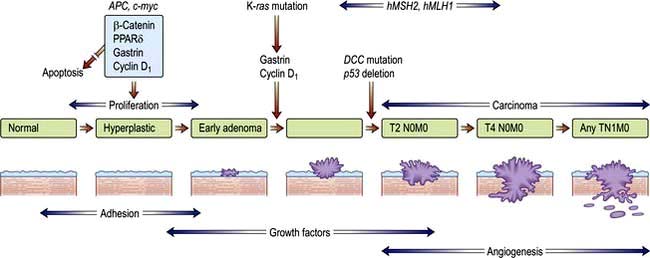
Most human neoplasms are clonal in origin, i.e. they arise from a single population of precursor or cancer stem cells. This process is typically initiated by genetic aberrations within this precursor cell. Cancer is increasingly common the older we get and can be related to a time dependent accumulation of DNA damage that is not repaired by the normal mechanisms of genome maintenance, damage tolerance and checkpoint pathways. Malignant transformation may result from a gain in function as cellular proto-oncogenes become mutated (e.g. ras), amplified (e.g. HER2) or translocated (e.g. BCR-ABL). However, these mutations are insufficient to cause malignant transformation by themselves. Alternatively, there may be a loss of function of tumour suppressor genes such as P53 that normally suppress growth. Loss or gain of function may also involve alterations in the genes controlling the transcription of the oncogenes or tumour suppressor genes (p. 46). Over subsequent cell divisions, heterogeneity develops with the accumulation of further genetic abnormalities (Fig. 9.1).
The genes most commonly affected can be characterized as those controlling cell cycle checkpoints, DNA repair and DNA damage recognition, apoptosis, differentiation, growth factor receptors and signalling pathways and tumour suppressor genes (Table 9.2). Recognition of critical genetic alterations has enabled extensive development of new targeted drugs such as imatinib that inhibits the growth signals of the abnormal tyrosine kinase BCR/ABL. Proliferation may continue at the expense of differentiation which, together with the failure of apoptosis, leads to tumour formation with the accumulation of morphologically abnormal cells varying in size, shape and cytoplasmic or nuclear maturity.
Table 9.2 Common genetic abnormalities in cancer
| Gene | Example |
|---|---|
Control cell cycle checkpoints | Cyclin D1, p15, p16 |
DNA repair | FANCA, ATM |
Apoptosis | Bcl2 |
Differentiation | PML/RARA |
Growth factor receptors | EGF, VEGF, FGF, BCR/ABL, TGF-B, KIT, L-FLT3 |
Signalling pathways | RAS, BRAF, JAK2, NF1, PTCH |
Hedgehog signalling pathway | See p. 26 |
Tumour suppressor genes | P53, Rb, WT1, VHL |
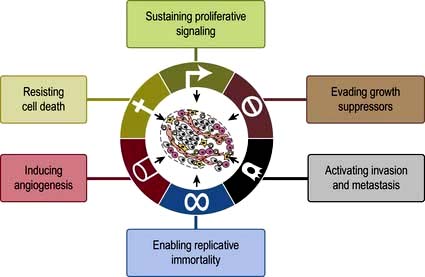
The hallmarks in developing cancer are shown in Figure 9.2.
Tumour immunology
Tumour cells are usually not recognized and killed by the immune system. There are two main reasons. The first is failure to express molecules such as HLA and co-stimulatory B7 molecules that are required for activation of cytotoxic, or ‘killer’, T lymphocytes. Second, tumours may also actively secrete immunosuppressive cytokines and cause a generalized immunosuppression. Successful strategies for tumour vaccines that overcome these obstacles are developing in renal cancer and prostate cancer. The monoclonal antibody ipilimumab against the inhibitory cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) molecule that is expressed after T-cell activation, is used in melanoma (p. 479)
Invasion and metastasis
Solid cancers spread by both local invasion and by distant metastasis through the vessels of the blood and lymphatic systems. Infiltration into surrounding tissues is associated with loss of cell–cell cohesion, which is mediated by active homotypic cell adhesion molecules (CAMs). Epithelial cadherin (E-cadherin) is expressed by many carcinomas and mutated in some such as familial gastric carcinoma (see p. 252).
Aetiology and epidemiology
Genetic factors
Rather than occurring by somatic mutation in response to mutagens, germline mutations in the genes that predispose to the development of cancer may be inherited and therefore present in all tissues. Examples of such cancer syndromes are given in Table 9.3. Expression of the mutation and hence carcinogenesis, will depend upon the penetrance (due to the level of expression and the presence of other genetic events) of the gene and whether the mutated allele has a dominant or recessive effect. There is a small group of autosomal dominant inherited mutations such as RB (in retinoblastoma), and a small group of recessive mutations (Table 9.3). Carriers of the recessive mutations are at risk of developing cancer if the second allele becomes mutated, leading to ‘loss of heterozygosity’ within the tumour, although this is seldom sufficient as carcinogenesis is a multistep process.
Table 9.3 Familial cancer syndromes
| Gene | Neoplasms | |
|---|---|---|
Autosomal dominant |
|
|
Retinoblastoma | RB1 | Eye |
Wilms’ tumour | WT1 | Kidney |
Li–Fraumeni | p53 | Sarcoma/brain/leukaemia |
Neurofibromatosis type 1 | NF1 | Neurofibromas/ leukaemia |
Familial adenomatous polyposis (FAP) | APC | Colon |
Hereditary non-polyposis colon cancer (HNPCC) | MLH1 and MSH2 | Colon, endometrium |
Hereditary diffuse gastric cancer syndrome | E-cadherin | Stomach |
Breast ovary families | BRCA1 | Breast/ovary |
BRCA2 | ||
p53 | ||
Melanoma | p16 | Skin |
Von Hippel–Lindau | VHL | Renal cell carcinoma and haemangioblastoma |
Multiple endocrine neoplasia type 1 | MEN1 | Pituitary, pancreas, parathyroid |
Multiple endocrine neoplasia type 2 | RET | Thyroid, adrenal medulla |
Autosomal recessive |
|
|
Xeroderma pigmentosa | XP | Skin |
Ataxia telangiectasia | AT | Leukaemia, lymphoma |
Fanconi’s anaemia | FA | Leukaemia, lymphoma |
Bloom’s syndrome | BS | Leukaemia, lymphoma |
Environmental factors
A wide range of environmental factors have been identified as being associated with the development of malignancy (Table 9.4) and may be amenable to preventative action such as smoking cessation, dietary modification and antiviral immunization (Box 9.1). Environmental factors interact with genetic predisposition. For example, subsequent generations of people moving from countries with a low incidence to those with a high incidence of breast or colon cancer acquire the cancer incidence of the country to which they have moved while northern European people exposed to strong UV radiation have the highest risk of developing melanoma.
Table 9.4 Some causative factors associated with the development of cancer
Smoking | Mouth, pharynx, oesophagus, larynx, lung, bladder, lip |
Alcohol | Mouth, pharynx, larynx, oesophagus, colorectal |
Iatrogenic |
|
Alkylating agents | Bladder, bone marrow |
Oestrogens | Endometrium, vagina, breast, cervix |
Androgens | Prostate |
Radiotherapy (e.g. mantle radiotherapy) | Carcinoma of breast and bronchus |
Diet |
|
High-fat diet | Colorectal cancer |
Environmental/occupation |
|
Vinyl chloride | Liver (angiosarcoma) |
Polycyclic hydrocarbons | Skin, lung, bladder, myeloid leukaemia |
Aromatic amines | Bladder |
Asbestos | Lung, mesothelium |
Ultraviolet light | Skin, lip |
Radiation | e.g. leukaemia, thyroid cancer |
Aflatoxin | Liver |
Biological agents |
|
Hepatitis B virus | Liver (hepatocellular carcinoma) |
Hepatitis C virus | Liver (hepatocellular carcinoma) |
Human T-cell leukaemia virus | Leukaemia/lymphoma |
Epstein–Barr virus | Burkitt’s lymphoma |
Hodgkin’s lymphoma | |
Nasopharyngeal carcinoma | |
Human papillomavirus types 16, 18 | Cervix |
Oral cancer (type 16) | |
Schistosoma japonicum | Bladder |
Helicobacter pylori | Stomach |
Tobacco
The incidence of lung cancer in both men and women increased dramatically in the last 25 years worldwide, but is now falling in many developed countries. The association of smoking with lung cancer is indisputable and causative mechanisms have been identified: cigarette tobacco is responsible for one-third of all deaths from cancer in the UK. Smoking not only causes lung cancer, it is also associated with cancer of the mouth, larynx, oesophagus and bladder. Smoking is discussed on page 806.
Diet
Dietary factors have been attributed to account for one-third of cancer deaths, although it is often difficult to differentiate these from other epidemiological factors. For example, the incidence of stomach cancer is particularly high in the Far East, while breast and colon cancers are more common in the Western, economically more developed countries. Many associations have been observed without a causative mechanism being identified between the incidence of cancer and the consumption of dietary fibre, red meat, saturated fats, salted fish, vitamin E, vitamin A and many others. Food and its role in the causation of gastrointestinal cancer is discussed in Chapter 5 (see p. 218). Increasing levels of obesity in the developed world have been associated with increases in women of cancers associated with oestrogenic stimulation of the breast and endometrium.
Infectious agents
Hepatocellular carcinoma occurs in patients with hepatitis B and C virus infections and Burkitt’s lymphoma and nasopharyngeal carcinoma are associated with the Epstein–Barr virus. EBV is also linked with Hodgkin’s lymphoma (see p. 459).
Radiation
Diagnostic. Imaging procedures involving radiation exposure are associated with an increased risk of cancer. This risk is cumulative, dose dependent and time dependent, i.e. children are at higher risk than adults. The cancer risk of various common investigations is shown in Table 9.5. All doctors should strive to minimize diagnostic exposure to radiation where possible using alternative modalities such as ultrasound or MRI. Good documentation of radiation doses is required. This is particularly so in children and pregnant women.
Table 9.5 Radiation exposure from common diagnostic radiological procedures
| Procedure | mSv |
|---|---|
CXR | 0.02 |
IVU | 3 |
CT chest | 7 |
CT abdomen | 8–10 |
Whole body CT | 20 |
Percutaneous coronary intervention | 15 |
Myocardial perfusion imaging | 15.6 |
UK background radiation is 2.6 mSv per year. 1 mSv carries a lifetime cancer risk of 1 in 17 500 and 5 mSv a risk of 1 in 3500.
Modified from: Smith-Bindman R, Lipson J, Marcus R et al. Archives of Internal Medicine 2009; 169:2078–2086 and Fazel R, Krumholz HM, Wang Y et al. New England Journal of Medicine 2009; 361:849–857.
Geographical distribution
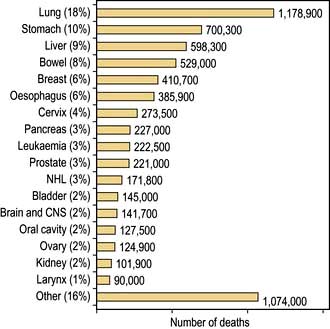
The incidence of cancer across the world is dependent on the local environmental factors, the diet and the genetics of the population (see above) (Figs 9.3, 9.4). Age is also a factor as most cancers occur in those over the age of 65 who comprise 3.3% of the population in Africa compared with 15.2% in Europe. Reproductive patterns also influence breast cancer. Migrating individuals often take on the risks of the local environmental factors.
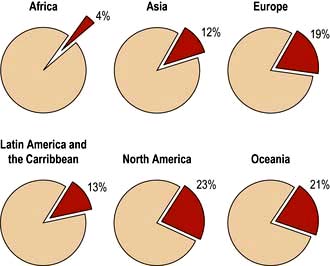
Figure 9.4 Percentage of all deaths due to cancer in the different regions of the world.
(From: http://info.canceresearchuk.org/cancerstats/world/the-global-picture/)
The clinical presentation of malignant disease
Asymptomatic detection through screening
Most common cancers start as focal microscopic clones of transformed cells and diagnosis only becomes likely once sufficient tumour bulk has accumulated to cause symptoms or signs. In order to try to make an earlier diagnosis and increase the curative possibilities, an increasing number of screening programmes are being developed which target the asymptomatic or preinvasive stages of the cancer as in cervix, breast and colon or use serum tumour markers as in prostate and ovarian cancers. Genetic screening can be used to target screening to groups at most risk of developing cancer, e.g. BRCA1 positive and breast cancer (see Table 9.3). The aim of screening programmes is to improve individual and/or population survival by detecting cancer at its very early stages when the patient is asymptomatic. This strategy is dependent upon finding tests that are sufficiently sensitive and specific, using detection methods that identify cancer before it has spread and having curative treatments that are practical and consistent with maintenance of a normal lifestyle and quality of life.
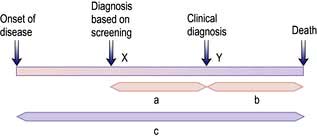
Unfortunately, earlier diagnosis does not necessarily mean longer survival and randomized trials are necessary to prove benefit. With lead time bias, the patient is merely treated at an earlier date and hence the survival appears longer; death still occurs at the same time from the point of genesis of the cancer (Fig. 9.5). With length time bias, a greater number of slowly growing tumours are detected when screening asymptomatic individuals leading to a false impression of an improvement in survival.
An effective screening procedure should:
 be affordable to the healthcare system
be affordable to the healthcare system
 be acceptable to all social groups so that they attend for screening
be acceptable to all social groups so that they attend for screening
 have a good discriminatory index between benign and malignant lesions
have a good discriminatory index between benign and malignant lesions
Colorectal cancer (CRC). Faecal occult blood is a cheap test for the detection of CRC. Large randomized studies have shown a reduction in cancer-related mortality of 15–33%. However, the false-positive rates are high, meaning many unnecessary colonoscopies (see p. 291). The UK has recently introduced a national screening programme using faecal occult blood in patients aged 60–64 years, in which positive tests have identified that 10% have cancer and 40% adenomas. A randomized trial in Norway has found an increased number of early stage cancers in the screened population but a high incidence of interval cancers between biennial screens.
Colonoscopy is the ‘gold-standard’ technique for the examination of the colon and rectum and is the investigation of choice for high-risk patients. Universal screening strategies have been recommended in the USA, but the shortage of skilled endoscopists, the expense, the need for full bowel preparation and the small risk of perforation make colonoscopy impractical as a population screening tool at present and CT colonography (‘virtual colonoscopy’) (see Fig. 6.5) may become an alternative along with genetic testing and stool DNA tests.
Other population-based screening programmes that are being used or are in trials are:
FURTHER READING
Menon U, Gentry-Maharaj A, Hallett R et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet. Oncology 2009; 10:327–340.
Paimela H, Malila N, Palva T et al. Early detection of colorectal cancer with faecal occult blood test screening. British Journal of Surgery 2010; 97:1567–1571.
Schroder FH et al. Prostate cancer mortality at 11 years of follow-up. N Engl J Med 2012; 366:981–990.
The symptomatic patient with cancer
Patients may offer information of predisposing conditions and family history that alerts the clinician to the likelihood of a cancer diagnosis. Many present with a history of tumour site-specific symptoms, e.g. pain, and physical signs, e.g. a mass, which readily identify the primary site of the cancer. However, some only seek medical attention when more systemic and nonspecific symptoms occur such as weight loss, night sweats, fever, fatigue, recurrent infections and anorexia. These usually indicate a more advanced stage of the disease, except in some paraneoplastic and ectopic endocrine syndromes (see below). Other patients are only diagnosed upon the discovery of established metastases such as the abdominal distension of ovarian cancer, the back pain of metastatic prostatic cancer or the liver enlargement of metastatic gastrointestinal cancer (Table 9.6).
Table 9.6 Symptoms and signs of malignant disease
| Degree of spread | Anatomical location | Examples of clinical problems |
|---|---|---|
Local | Mass | Thyroid nodule, pigmented naevus, breast lump, abdominal mass, testicular mass |
Local infiltration of skin | Dermal nodules, peau d’orange, ulceration | |
Local infiltration of nerve | Neuropathic pain and loss of function | |
Local infiltration of vessel | Venous thrombosis, tumour emboli, haemorrhage, e.g. GI | |
Obstruction of viscera or duct | Small or large bowel obstruction, dysphagia, SVC obstruction | |
Nodal | Peripheral | Supraclavicular fossa, Virchow’s node, lymphoedema |
Central | Mediastinum – SVC obstruction, porta hepatis – obstructive jaundice, para-aortic nodes and back pain | |
Metastatic | Lung | Pleuritic pain, cough, shortness of breath, lymphangitis and respiratory failure, recurrent pneumonia |
Liver | RUQ pain, anorexia, fever, raised serum liver enzymes, jaundice | |
Brain | Headache and vomiting of raised intracranial pressure, focal deficit, coma, seizure | |
Bone | Bone pain, cord compression, fracture, hypercalcaemia | |
Pleura | Effusion, pain, shortness of breath | |
Peritoneum | Ascites, Krukenberg tumours | |
Adrenal | Addison’s disease (hypoadrenalism) | |
Umbilicus | Sister Mary Joseph’s nodule |
Paraneoplastic syndromes are indirect effects of cancer (Box 9.2, Fig. 9.6) that are often associated with specific types of cancer and may be reversible with treatment of the cancer. The effects and mechanisms can be very variable. For example in the Lambert–Eaton syndrome (see p. 1152), there is cross-reactivity between tumour antigens and the normal tissues, e.g. the acetylcholine receptors at neuromuscular junctions.
![]() Box 9.2
Box 9.2
Paraneoplastic syndromes
| Syndrome | Tumour | Serum antibodies |
|---|---|---|
Neurological |
|
|
Lambert–Eaton syndrome | Lung (small-cell) lymphoma | Anti-VGLC |
Peripheral sensory neuropathy | Lung (small-cell), breast and ovary lymphoma | Anti-Hu |
Cerebellar degeneration | Lung (particularly small-cell) lymphoma | Anti-Yo |
Opsoclonus/myoclonus | Breast, lung (small-cell) | Anti-Ri |
Stiff person syndrome | Breast, lung (small-cell) | Anti-amphiphysin |
Limbic, hypothalamic, brain stem encephalitis | Lung | Anti-Ma protein |
| Testicular | Anti-NMDAR |
Endocrine/metabolic |
|
|
SIADH | Lung (small-cell) |
|
Ectopic ACTH secretion | Lung (small-cell) |
|
Hypercalcaemia | Renal, breast, myeloma, lymphoma |
|
Fever | Lymphoma, renal |
|
Musculoskeletal |
|
|
Hypertrophic pulmonary osteoarthropathy | Lung (non-small-cell) |
|
Clubbing | Lung |
|
Skin |
|
|
Dermatomyositis/polymyositis | Lung and upper GI |
|
Acanthosis nigricans | Mainly gastric |
|
Velvet palms | Gastric, lung (non-small cell) |
|
Hyperpigmentation | Lung (small-cell) |
|
Pemphigus | Non-Hodgkin’s lymphoma, CLL |
|
Haematological |
|
|
Erythrocytosis | Renal cell carcinoma, hepatocellular carcinoma, cerebellar haemangioblastoma |
|
Thrombocytosis | Ovarian cancer |
|
Migratory thrombophlebitis | Pancreatic adenocarcinoma |
|
DVT | Adenocarcinoma |
|
DIC | Adenocarcinoma |
|
Renal |
|
|
Nephrotic syndrome | Myeloma, amyloidosis |
|
Membranous glomerulonephritis | Lymphoma |
|
SIADH, syndrome of inappropriate antidiuretic hormone secretion; ACTH, adrenocorticotrophic hormone; CLL, chronic lymphocytic leukaemia; DIC, disseminated intravascular coagulation; NMDAR, N-methyl-D-aspartate receptors.
The coagulopathy of cancer may present with thrombophlebitis, deep venous thrombosis and pulmonary emboli, particularly in association with cancers of pancreas, stomach and breast. Some 18% of patients with recurrent pulmonary embolus will be found to have an underlying cancer and many cancer patients are at increased risk of venous thromboembolism (VTE) following diagnosis. Trousseau’s syndrome – superficial thrombophlebitis migrans – refers to this process in the superficial venous system. All patients with active cancer admitted to hospital are at high risk of VTE and should be given prophylaxis with subcutaneous LMW heparin in the absence of any contraindications (see p. 429). Dabigatran, an oral direct thrombin inhibitor, is an alternative therapy.
Other symptoms are related to peptide or hormone release, e.g. carcinoid or Cushing’s syndrome.
Serum tumour markers
Tumour markers are intracellular proteins or cell surface glycoproteins released into the circulation and detected by immunoassays. Examples are given in Table 9.7. Values in the normal range do not necessarily equate with the absence of disease and a positive result must be corroborated by histology as these markers can be seen in many benign conditions. They are most useful in the serial monitoring of response to treatment. As discussed in subsequent sections, a proportion of low-grade B-cell lymphomas and a majority of cases of myeloma will produce a monoclonal paraprotein of intact immunoglobulin molecule or light chains. This acts as a valuable tumour marker in the diagnosis and assessment of response.
Table 9.7 Serum tumour markers
α-Fetoprotein | Hepatocellular carcinoma and non-seminomatous germ cell tumours of the gonads |
β-Human chorionic gonadotrophin (β-hCG) | Choriocarcinomas, germ cell tumours (testicular) and lung cancers |
Prostate-specific antigen (PSA) | Carcinoma of prostate |
Carcinoma embryonic antigen (CEA) | Gastrointestinal cancers |
CA125 | Ovarian cancer |
CA19–9 | Gastrointestinal cancers, particularly pancreatic cancer |
CA15–3 | Breast cancer |
Osteopontin | Many cancers including mesothelioma |
M-band (Ig or light chain) | Myeloma, chronic lymphocytic leukaemia, small lymphocytic lymphoma, lymphoplasmacytic lymphoma, amyloid |
Biopsy and histological examination
Molecular markers of genetic abnormalities have long been available in the haematological cancers and are increasingly available in solid cancers. For example, fluorescent in situ hybridization (FISH, see p. 40) can be used to look for characteristic chromosomal translocations, e.g. in lymphoma and leukaemia, as well as deletions or amplifications, e.g. in breast cancer (see genetic basis of cancer, p. 45). Tissue microarrays can identify patterns of multiple genomic alterations and single nucleotide polymorphisms (SNPs), e.g. in breast cancer and lymphoma (see p. 35), and RNA assays with RT-PCR can be used to identify tissue of origin with prognostic and predictive relevance.
Cancer treatment
Aims of treatment
The organization across multiple departments and coordination from primary to secondary and tertiary care has become known as a patient pathway. Establishment of agreed patient pathways has enabled more effective and timely delivery of care and post-treatment rehabilitation. The aim is to provide optimal treatment and for the patient to experience seamless and high quality care and to allow audit and continuing improvement against agreed standards. Central to this endeavour is the involvement of the patient, through education as to the nature of their disease and the treatment options available. An informed choice can then be made, even if in the end it is simply to abide by the decisions made by the professionals. Good communication embodies a humane approach which preserves hope at an appropriate level through empathy and understanding of the patient’s position (see p. 14).