FIGURE 105-4 Influence of vismodegib on the hedgehog (Hh) pathway. Normally, one of three Hh ligands (sonic [SHh], Indian, or desert) binds to patched homolog 1 (PTCH1), causing its degradation and release of smoothened homolog (SMO). The downstream events of SMO release are the activation of Gli1, Gli2, and Gli3 through the transcriptional regulator known as SUFU. Gli1 and Gli2 translocate to the nucleus and promote gene transcription. Vismodegib is an SMO antagonist that decreases the interaction between SMO and PTCH1, resulting in decreased Hh pathway signaling, gene transcription, and cell division. The downstream Hh pathway events inhibited by vismodegib are indicated in red.
CLINICAL PRESENTATION
Basal Cell Carcinoma BCC arises from epidermal basal cells. The least invasive of BCC subtypes, superficial BCC, consists of often subtle, erythematous scaling plaques that slowly enlarge and are most commonly seen on the trunk and proximal extremities (Fig. 105-5). This BCC subtype may be confused with benign inflammatory dermatoses, especially nummular eczema and psoriasis. BCC also can present as a small, slowly growing pearly nodule, often with tortuous telangiectatic vessels on its surface, rolled borders, and a central crust (nodular BCC). The occasional presence of melanin in this variant of nodular BCC (pigmented BCC) may lead to confusion with melanoma. Morpheaform (fibrosing), infiltrative, and micronodular BCC, the most invasive and potentially aggressive subtypes, manifest as solitary, flat or slightly depressed, indurated whitish, yellowish, or pink scar-like plaques. Borders are typically indistinct, and lesions can be subtle; thus, delay in treatment is common, and tumors can be more extensive than expected clinically.
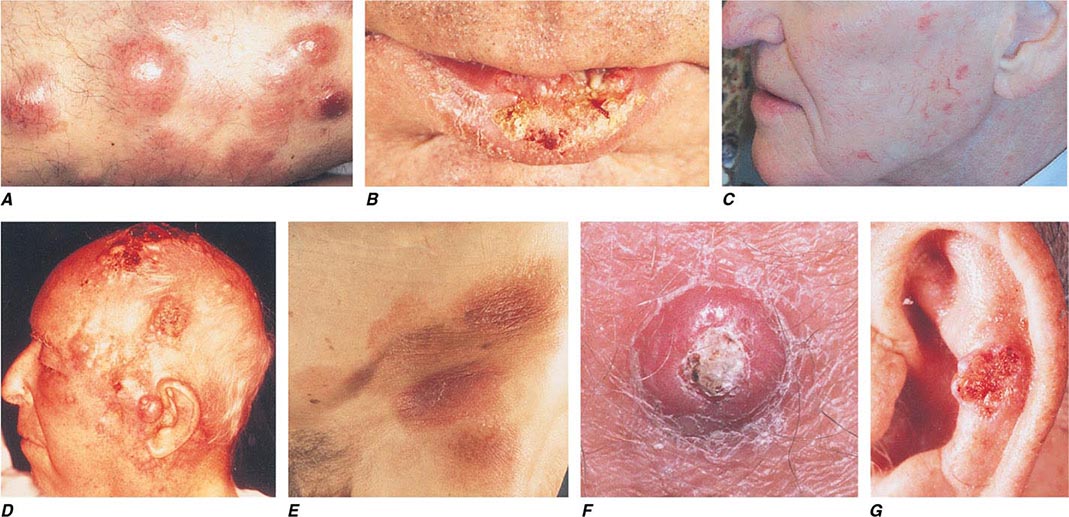
FIGURE 105-5 Cutaneous neoplasms. A. Non-Hodgkin’s lymphoma involves the skin with typical violaceous, “plum-colored” nodules. B. Squamous cell carcinoma is seen here as a hyperkeratotic crusted and somewhat eroded plaque on the lower lip. Sun-exposed skin in areas such as the head, neck, hands, and arms represent other typical sites of involvement. C. Actinic keratoses consist of hyperkeratotic erythematous papules and patches on sun-exposed skin. They arise in middle-aged to older adults and have some potential for malignant transformation. D. Metastatic carcinoma to the skin is characterized by inflammatory, often ulcerated dermal nodules. E. Mycosis fungoides is a cutaneous T cell lymphoma, and plaque-stage lesions are seen in this patient. F. Keratoacanthoma is a low-grade squamous cell carcinoma that presents as an exophytic nodule with central keratinous debris. G. This basal cell carcinoma shows central ulceration and a pearly, rolled telangiectatic tumor border.
Squamous Cell Carcinoma Primary cutaneous SCC is a malignant neoplasm of keratinizing epidermal cells. SCC has a variable clinical course, ranging from indolent to rapid growth kinetics, with the potential for metastasis to regional and distant sites. Commonly, SCC appears as an ulcerated erythematous nodule or superficial erosion on sun-exposed skin of the head, neck, trunk, and extremities (Fig. 105-5). It may also appear as a banal, firm, dome-shaped papule or rough-textured plaque. It is commonly mistaken for a wart or callous when the inflammatory response to the lesion is minimal. Clinically visible overlying telangiectasias are uncommon, although dotted or coiled vessels are a hallmark of SCC when viewed through a dermatoscope. The margins of this tumor may be ill defined, and fixation to underlying structures may occur (“tethering”).
A very rapidly growing but low-grade form of SCC, called keratoacanthoma (KA), typically appears as a large dome-shaped papule with a central keratotic crater. Some KAs regress spontaneously without therapy, but because progression to metastatic SCC has been documented, KAs should be treated in the same manner as other types of cutaneous SCC. KAs are also associated with medications that target BRAF mutations and occur in 15–25% of patients receiving these medications.
Actinic keratoses and cheilitis (actinic keratoses occurring on the lip), both premalignant forms of SCC, present as hyperkeratotic papules on sun-exposed areas. The potential for malignant degeneration in untreated lesions ranges from 0.25 to 20%. SCC in situ, also called Bowen’s disease, is the intraepidermal form of SCC and usually presents as a scaling, erythematous plaque. As with invasive SCC, SCC in situ most commonly arises on sun-damaged skin, but can occur anywhere on the body. Bowen’s disease forming secondary to infection with human papillomavirus (HPV) can arise on skin with minimal or no prior sun exposure, such as the buttock or posterior thigh. Treatment of premalignant and in situ lesions reduces the subsequent risk of invasive disease.
NATURAL HISTORY
Basal Cell Carcinoma The natural history of BCC is that of a slowly enlarging, locally invasive neoplasm. The degree of local destruction and risk of recurrence vary with the size, duration, location, and histologic subtype of the tumor. Location on the central face, ears, or scalp may portend a higher risk. Small nodular, pigmented, cystic, or superficial BCCs respond well to most treatments. Large lesions and micronodular, infiltrative, and morpheaform subtypes may be more aggressive. The metastatic potential of BCC is low (0.0028–0.1% in immunocompetent patients), but the risk of recurrence or a new primary NMSC is about 40% over 5 years.
Squamous Cell Carcinoma The natural history of SCC depends on tumor and host characteristics. Tumors arising on sun-damaged skin have a lower metastatic potential than do those on non-sun-exposed areas. Cutaneous SCC metastasizes in 0.3–5.2% of individuals, most frequently to regional lymph nodes. Tumors occurring on the lower lip and ear develop regional metastases in 13 and 11% of patients, respectively, whereas the metastatic potential of SCC arising in scars, chronic ulcerations, and genital or mucosal surfaces is higher. Recurrent SCC has a much higher potential for metastatic disease, approaching 30%. Large, poorly differentiated, deep tumors with perineural or lymphatic invasion, multifocal tumors, and those arising in immunosuppressed patients often behave aggressively.
PREVENTION
The general principles for prevention are those described for melanoma earlier. Unique strategies for NMSC include active surveillance for patients on immunosuppressive medications or BRAF-targeted therapy. Chemoprophylaxis using synthetic retinoids and immunosuppression reduction when possible may be useful in controlling new lesions and managing patients with multiple tumors.
OTHER NONMELANOMA CUTANEOUS MALIGNANCIES
Neoplasms of cutaneous adnexae and sarcomas of fibrous, mesenchymal, fatty, and vascular tissues make up the remaining 1–2% of NMSCs.
Merkel cell carcinoma (MCC) is a neural crest–derived highly aggressive malignancy with mortality rates approaching 33% at 3 years. An oncogenic Merkel cell polyomavirus is present in 80% of tumors. Many patients have detectable cellular or humoral immune responses to polyoma viral proteins, although this immune response is insufficient to eradicate the malignancy. Survival depends on extent of disease: 90% survive with local disease, 52% with nodal involvement, and only 10% with distant disease at 3 years. MCC incidence tripled over the last 20 years with an estimated 1600 cases per year in the United States. Immunosuppression can increase incidence and diminish prognosis. MCC lesions typically present as an asymptomatic rapidly expanding bluish-red/violaceous tumor on sun-exposed skin of older white patients. Treatment is surgical excision with sentinel lymph node biopsy for accurate staging in patients with localized disease, often followed by adjuvant RT. Patients with extensive disease can be offered systemic chemotherapy; however, there is no convincing survival benefit. Whenever possible a clinical trial should be considered for this rare but aggressive NMSC, especially in light of the potential for new treatments directed at the oncogenic virus that causes this malignancy.
Extramammary Paget’s disease is an uncommon apocrine malignancy arising from stem cells of the epidermis that are characterized histologically by the presence of Paget cells. These tumors present as moist erythematous patches on anogenital or axillary skin of the elderly. Outcomes are generally good with site-directed surgery, and 5-year disease specific survival is approximately 95% with localized disease. Advanced age and extensive disease at presentation are factors that confer diminished prognosis. RT or topical imiquimod can be considered for more extensive disease. Local management may be challenging because these tumors often extend far beyond clinical margins; surgical excision with MMS has the highest cure rates. Similarly, MMS is the treatment of choice in other rare cutaneous tumors with extensive subclinical extension such as dermatofibromasarcoma protuberans.
Kaposi’s sarcoma (KS) is a soft tissue sarcoma of vascular origin that is induced by the human herpesvirus 8. The incidence of KS increased dramatically during the AIDS epidemic, but has now decreased tenfold with the institution of highly active antiretroviral therapy.
ACKNOWLEDGMENT
Carl V. Washington, MD, and Hari Nadiminti, MD, contributed to this chapter in the 18th edition, and material from that chapter is included here. Claudia Taylor, MD, and Steven Kolker, MD, provided valued feedback and suggested many improvements to this chapter.
106 | Head and Neck Cancer |
Epithelial carcinomas of the head and neck arise from the mucosal surfaces in the head and neck and typically are squamous cell in origin. This category includes tumors of the paranasal sinuses, the oral cavity, and the nasopharynx, oropharynx, hypopharynx, and larynx. Tumors of the salivary glands differ from the more common carcinomas of the head and neck in etiology, histopathology, clinical presentation, and therapy. They are rare and histologically highly heterogeneous. Thyroid malignancies are described in Chap. 405.
INCIDENCE AND EPIDEMIOLOGY
![]() The number of new cases of head and neck cancers (oral cavity, pharynx, and larynx) in the United States was 53,640 in 2013, accounting for about 3% of adult malignancies; 11,520 people died from the disease. The worldwide incidence exceeds half a million cases annually. In North America and Europe, the tumors usually arise from the oral cavity, oropharynx, or larynx. The incidence of oropharyngeal cancers is increasing in recent years. Nasopharyngeal cancer is more commonly seen in the Mediterranean countries and in the Far East, where it is endemic in some areas.
The number of new cases of head and neck cancers (oral cavity, pharynx, and larynx) in the United States was 53,640 in 2013, accounting for about 3% of adult malignancies; 11,520 people died from the disease. The worldwide incidence exceeds half a million cases annually. In North America and Europe, the tumors usually arise from the oral cavity, oropharynx, or larynx. The incidence of oropharyngeal cancers is increasing in recent years. Nasopharyngeal cancer is more commonly seen in the Mediterranean countries and in the Far East, where it is endemic in some areas.
ETIOLOGY AND GENETICS
Alcohol and tobacco use are the most significant risk factors for head and neck cancer, and when used together, they act synergistically. Smokeless tobacco is an etiologic agent for oral cancers. Other potential carcinogens include marijuana and occupational exposures such as nickel refining, exposure to textile fibers, and woodworking.
Some head and neck cancers have a viral etiology. Epstein-Barr virus (EBV) infection is frequently associated with nasopharyngeal cancer, especially in endemic areas of the Mediterranean and Far East. EBV antibody titers can be measured to screen high-risk populations. Nasopharyngeal cancer has also been associated with consumption of salted fish and in-door pollution.
In Western countries, the human papilloma virus (HPV) is associated with a rising incidence of tumors arising from the oropharynx, i.e., the tonsillar bed and base of tongue. Over 50% of oropharyngeal tumors are caused by HPV in the United States. HPV 16 is the dominant viral subtype, although HPV 18 and other oncogenic subtypes are seen as well. Alcohol- and tobacco-related cancers, on the other hand, have decreased in incidence. HPV-related oropharyngeal cancer occurs in a younger patient population and is associated with increased numbers of sexual partners and oral sexual practices. It is associated with a better prognosis, especially for nonsmokers.
Dietary factors may contribute. The incidence of head and neck cancer is higher in people with the lowest consumption of fruits and vegetables. Certain vitamins, including carotenoids, may be protective if included in a balanced diet. Supplements of retinoids, such as cis-retinoic acid, have not been shown to prevent head and neck cancers (or lung cancer) and may increase the risk in active smokers. No specific risk factors or environmental carcinogens have been identified for salivary gland tumors.
HISTOPATHOLOGY, CARCINOGENESIS, AND MOLECULAR BIOLOGY
Squamous cell head and neck cancers are divided into well-differentiated, moderately well-differentiated, and poorly differentiated categories. Poorly differentiated tumors have a worse prognosis than well-differentiated tumors. For nasopharyngeal cancers, the less common differentiated squamous cell carcinoma is distinguished from nonker-atinizing and undifferentiated carcinoma (lymphoepithelioma) that contains infiltrating lymphocytes and is commonly associated with EBV.
Salivary gland tumors can arise from the major (parotid, submandibular, sublingual) or minor salivary glands (located in the submucosa of the upper aerodigestive tract). Most parotid tumors are benign, but half of submandibular and sublingual gland tumors and most minor salivary gland tumors are malignant. Malignant tumors include mucoepidermoid and adenoid cystic carcinomas and adenocarcinomas.
The mucosal surface of the entire pharynx is exposed to alcohol- and tobacco-related carcinogens and is at risk for the development of a premalignant or malignant lesion. Erythroplakia (a red patch) or leukoplakia (a white patch) can be histopathologically classified as hyperplasia, dysplasia, carcinoma in situ, or carcinoma. However, most head and neck cancer patients do not present with a history of premalignant lesions. Multiple synchronous or metachronous cancers can also be observed. In fact, over time, patients with early-stage head and neck cancer are at greater risk of dying from a second malignancy than from a recurrence of the primary disease.
Second head and neck malignancies are usually not therapy-induced; they reflect the exposure of the upper aerodigestive mucosa to the same carcinogens that caused the first cancer. These second primaries develop in the head and neck area, the lung, or the esophagus. Thus, computed tomography (CT) screening for lung cancer in heavy smokers who have already developed a head and neck cancer should be considered. Rarely, patients can develop a radiation therapy–induced sarcoma after having undergone prior radiotherapy for a head and neck cancer.
Much progress has been made in describing the molecular features of head and neck cancer. These features have allowed investigators to describe the genetic and epigenetic alterations and the mutational spectrum of these tumors. Early reports demonstrated frequent overexpression of the epidermal growth factor receptor (EGFR). Overexpression was shown to correlate with poor prognosis. However, it has not proved to be a good predictor of tumor response to EGFR inhibitors, which are successful in only about 10–15% of patients. p53 mutations are also found frequently with other major affected oncogenic driver pathways including the mitotic signaling and Notch pathways and cell cycle regulation. The PI3K pathway is frequently altered, especially in HPV-positive tumors, where it is the only mutated cancer gene identified to date. Overall, these alterations affect mitogenic signaling, genetic stability, cellular proliferation, and differentiation. HPV is known to act through inhibition of the p53 and RB tumor-suppressor genes, thereby initiating the carcinogenic process, and has a mutational spectrum distinct from alcohol- and tobacco-related tumors.
CLINICAL PRESENTATION AND DIFFERENTIAL DIAGNOSIS
Most tobacco-related head and neck cancers occur in patients older than age 60 years. HPV-related malignancies are frequently diagnosed in younger patients, usually in their forties or fifties, whereas EBV-related nasopharyngeal cancer can occur in all ages, including teenagers. The manifestations vary according to the stage and primary site of the tumor. Patients with nonspecific signs and symptoms in the head and neck area should be evaluated with a thorough otolaryngologic exam, particularly if symptoms persist longer than 2–4 weeks. Males are more frequently affected than women by head and neck cancers, including HPV-positive tumors.
Cancer of the nasopharynx typically does not cause early symptoms. However, it may cause unilateral serous otitis media due to obstruction of the eustachian tube, unilateral or bilateral nasal obstruction, or epistaxis. Advanced nasopharyngeal carcinoma causes neuropathies of the cranial nerves due to skull base involvement.
Carcinomas of the oral cavity present as nonhealing ulcers, changes in the fit of dentures, or painful lesions. Tumors of the tongue base or oropharynx can cause decreased tongue mobility and alterations in speech. Cancers of the oropharynx or hypopharynx rarely cause early symptoms, but they may cause sore throat and/or otalgia. HPV-related tumors frequently present with neck lymphadenopathy as the first sign.
Hoarseness may be an early symptom of laryngeal cancer, and persistent hoarseness requires referral to a specialist for indirect laryngoscopy and/or radiographic studies. If a head and neck lesion treated initially with antibiotics does not resolve in a short period, further workup is indicated; to simply continue the antibiotic treatment may be to lose the chance of early diagnosis of a malignancy.
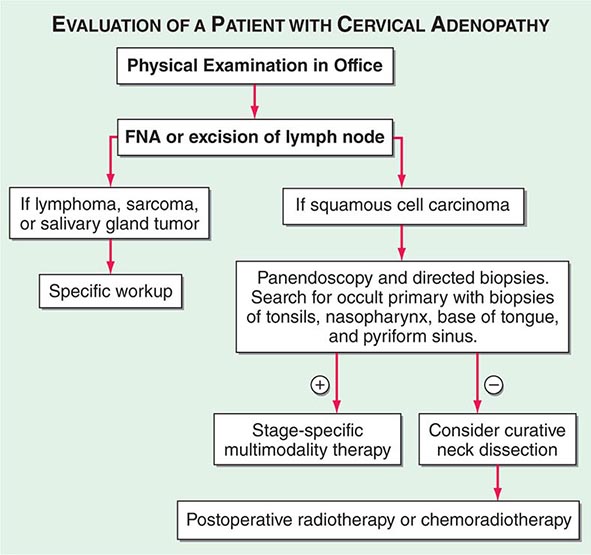
Advanced head and neck cancers in any location can cause severe pain, otalgia, airway obstruction, cranial neuropathies, trismus, odynophagia, dysphagia, decreased tongue mobility, fistulas, skin involvement, and massive cervical lymphadenopathy, which may be unilateral or bilateral. Some patients have enlarged lymph nodes even though no primary lesion can be detected by endoscopy or biopsy; these patients are considered to have carcinoma of unknown primary (Fig. 106-1). If the enlarged nodes are located in the upper neck and the tumor cells are of squamous cell histology, the malignancy probably arose from a mucosal surface in the head or neck. Tumor cells in supraclavicular lymph nodes may also arise from a primary site in the chest or abdomen.
FIGURE 106-1 Evaluation of a patient with cervical adenopathy without a primary mucosal lesion; a diagnostic workup. FNA, fine-needle aspiration.
The physical examination should include inspection of all visible mucosal surfaces and palpation of the floor of the mouth and of the tongue and neck. In addition to tumors themselves, leukoplakia (a white mucosal patch) or erythroplakia (a red mucosal patch) may be observed; these “premalignant” lesions can represent hyperplasia, dysplasia, or carcinoma in situ and require biopsy. Further examination should be performed by a specialist. Additional staging procedures include CT of the head and neck to identify the extent of the disease. Patients with lymph node involvement should have CT scan of the chest and upper abdomen to screen for distant metastases. In heavy smokers, the CT scan of the chest can also serve as a screening tool to rule out a second lung primary tumor. A positron emission tomography (PET) scan may also be administered and can help to identify or exclude distant metastases. The definitive staging procedure is an endoscopic examination under anesthesia, which may include laryngoscopy, esophagoscopy, and bronchoscopy; during this procedure, multiple biopsy samples are obtained to establish a primary diagnosis, define the extent of primary disease, and identify any additional premalignant lesions or second primaries.
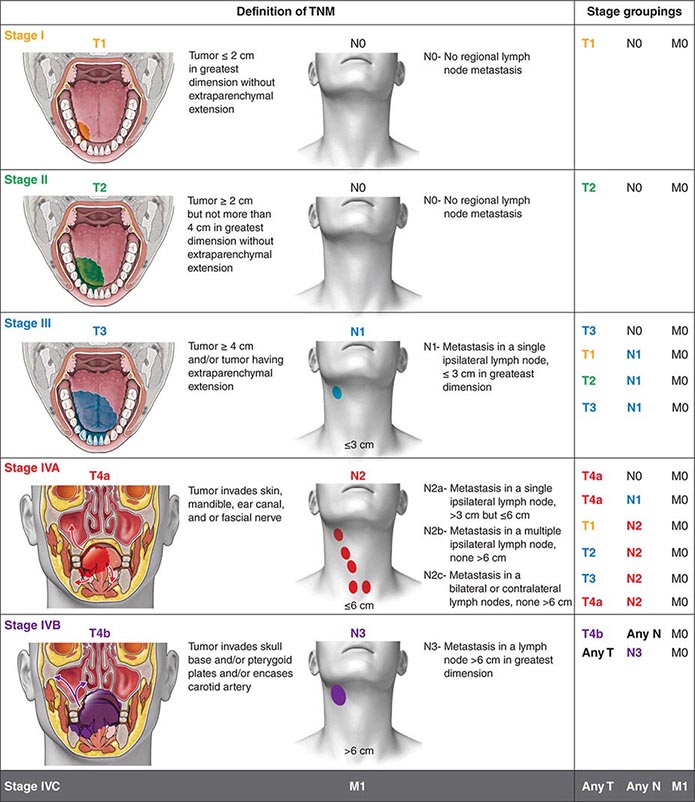
Head and neck tumors are classified according to the tumor-node-metastasis (TNM) system of the American Joint Committee on Cancer (Fig. 106-2). This classification varies according to the specific anatomic subsite. In general, primary tumors are classified as T1 to T3 by increasing size, whereas T4 usually represents invasion of another structure such as bone, muscle, or root of tongue. Lymph nodes are staged by size, number, and location (ipsilateral vs contralateral to the primary). Distant metastases are found in <10% of patients at initial diagnosis and are more common in patients with advanced lymph node stage; microscopic involvement of the lungs, bones, or liver is more common, particularly in patients with advanced neck lymph node disease. Modern imaging techniques may increase the number of patients with clinically detectable distant metastases in the future.
FIGURE 106-2 Tumor-node-metastasis (TNM) staging system.
In patients with lymph node involvement and no visible primary, the diagnosis should be made by lymph node excision (Fig. 106-1). If the results indicate squamous cell carcinoma, a panendoscopy should be performed, with biopsy of all suspicious-appearing areas and directed biopsies of common primary sites, such as the nasopharynx, tonsil, tongue base, and pyriform sinus. HPV-positive tumors especially can have small primary tumors that spread early to locoregional lymph nodes.
SALIVARY GLAND TUMORS
Most benign salivary gland tumors are treated with surgical excision, and patients with invasive salivary gland tumors are treated with surgery and radiation therapy. These tumors may recur regionally; adenoid cystic carcinoma has a tendency to recur along the nerve tracks. Distant metastases may occur as late as 10–20 years after the initial diagnosis. For metastatic disease, therapy is given with palliative intent, usually chemotherapy with doxorubicin and/or cisplatin. Identification of novel agents with activity in these tumors is a high priority.
107 | Neoplasms of the Lung |
Lung cancer, which was rare prior to 1900 with fewer than 400 cases described in the medical literature, is considered a disease of modern man. By the mid-twentieth century, lung cancer had become epidemic and firmly established as the leading cause of cancer-related death in North America and Europe, killing over three times as many men as prostate cancer and nearly twice as many women as breast cancer. This fact is particularly troubling because lung cancer is one of the most preventable of all of the major malignancies. Tobacco consumption is the primary cause of lung cancer, a reality firmly established in the mid-twentieth century and codified with the release of the U.S. Surgeon General’s 1964 report on the health effects of tobacco smoking. Following the report, cigarette use started to decline in North America and parts of Europe, and with it, so did the incidence of lung cancer. To date, the decline in lung cancer is seen most clearly in men; only recently has the decline become apparent among women in the United States. Unfortunately, in many parts of the world, especially in countries with developing economies, cigarette use continues to increase, and along with it, the incidence of lung cancers is also rising. Although tobacco smoking remains the primary cause of lung cancer worldwide, approximately 60% of new lung cancers in the United States occur in former smokers (smoked ≥100 cigarettes per lifetime, quit ≥1 year), many of whom quit decades ago, or never smokers (smoked <100 cigarettes per lifetime). Moreover, one in five women and one in 12 men diagnosed with lung cancer have never smoked. Given the magnitude of the problem, it is incumbent that every internist has a general knowledge of lung cancer and its management.
EPIDEMIOLOGY
Lung cancer is the most common cause of cancer death among American men and women. More than 225,000 individuals will be diagnosed with lung cancer in the United States in 2013, and over 150,000 individuals will die from the disease. The incidence of lung cancer peaked among men in the late 1980s and has plateaued in women. Lung cancer is rare below age 40, with rates increasing until age 80, after which the rate tapers off. The projected lifetime probability of developing lung cancer is estimated to be approximately 8% among males and approximately 6% among females. The incidence of lung cancer varies by racial and ethnic group, with the highest age-adjusted incidence rates among African Americans. The excess in age-adjusted rates among African Americans occurs only among men, but examinations of age-specific rates show that below age 50, mortality from lung cancer is more than 25% higher among African American than Caucasian women. Incidence and mortality rates among Hispanics and Native and Asian Americans are approximately 40–50% those of whites.
RISK FACTORS
Cigarette smokers have a 10-fold or greater increased risk of developing lung cancer compared to those who have never smoked. A deep sequencing study suggested that one genetic mutation is induced for every 15 cigarettes smoked. The risk of lung cancer is lower among persons who quit smoking than among those who continue smoking; former smokers have a ninefold increased risk of developing lung cancer compared to men who have never smoked versus the 20-fold excess in those who continue to smoke. The size of the risk reduction increases with the length of time the person has quit smoking, although generally even long-term former smokers have higher risks of lung cancer than those who never smoked. Cigarette smoking has been shown to increase the risk of all the major lung cancer cell types. Environmental tobacco smoke (ETS) or second-hand smoke is also an established cause of lung cancer. The risk from ETS is less than from active smoking, with about a 20–30% increase in lung cancer observed among never smokers married for many years to smokers, in comparison to the 2000% increase among continuing active smokers.
Although cigarette smoking is the cause of the majority of lung cancers, several other risk factors have been identified, including occupational exposures to asbestos, arsenic, bischloromethyl ether, hexavalent chromium, mustard gas, nickel (as in certain nickel-refining processes), and polycyclic aromatic hydrocarbons. Occupational observations also have provided insight into possible mechanisms of lung cancer induction. For example, the risk of lung cancer among asbestos-exposed workers is increased primarily among those with underlying asbestosis, raising the possibility that the scarring and inflammation produced by this fibrotic nonmalignant lung disease may in many cases (although likely not in all) be the trigger for asbestos-induced lung cancer. Several other occupational exposures have been associated with increased rates of lung cancer, but the causal nature of the association is not as clear.
The risk of lung cancer appears to be higher among individuals with low fruit and vegetable intake during adulthood. This observation led to hypotheses that specific nutrients, in particular retinoids and carotenoids, might have chemopreventative effects for lung cancer. However, randomized trials failed to validate this hypothesis. In fact, studies found the incidence of lung cancer was increased among smokers with supplementation. Ionizing radiation is also an established lung carcinogen, most convincingly demonstrated from studies showing increased rates of lung cancer among survivors of the atom bombs dropped on Hiroshima and Nagasaki and large excesses among workers exposed to alpha irradiation from radon in underground uranium mining. Prolonged exposure to low-level radon in homes might impart a risk of lung cancer equal or greater than that of ETS. Prior lung diseases such as chronic bronchitis, emphysema, and tuberculosis have been linked to increased risks of lung cancer as well.
Smoking Cessation Given the undeniable link between cigarette smoking and lung cancer (not even addressing other tobacco-related illnesses), physicians must promote tobacco abstinence. Physicians also must help their patients who smoke to stop smoking. Smoking cessation, even well into middle age, can minimize an individual’s subsequent risk of lung cancer. Stopping tobacco use before middle age avoids more than 90% of the lung cancer risk attributable to tobacco. However, there is little health benefit derived from just “cutting back.” Importantly, smoking cessation can even be beneficial in individuals with an established diagnosis of lung cancer, as it is associated with improved survival, fewer side effects from therapy, and an overall improvement in quality of life. Moreover, smoking can alter the metabolism of many chemotherapy drugs, potentially adversely altering the toxicities and therapeutic benefits of the agents. Consequently, it is important to promote smoking cessation even after the diagnosis of lung cancer is established.
Physicians need to understand the essential elements of smoking cessation therapy. The individual must want to stop smoking and must be willing to work hard to achieve the goal of smoking abstinence. Self-help strategies alone only marginally affect quit rates, whereas individual and combined pharmacotherapies in combination with counseling can significantly increase rates of cessation. Therapy with an antidepressant (e.g., bupropion) and nicotine replacement therapy (varenicline, a α4β2 nicotinic acetylcholine receptor partial agonist) are approved by the U.S. Food and Drug Administration (FDA) as first-line treatments for nicotine dependence. However, both drugs have been reported to increase suicidal ideation and must be used with caution. In a randomized trial, varenicline was shown to be more efficacious than bupropion or placebo. Prolonged use of varenicline beyond the initial induction phase proved useful in maintaining smoking abstinence. Clonidine and nortriptyline are recommended as second-line treatments. Of note, reducing cigarettes smoked before quit day and quitting abruptly, with no prior reduction, yield comparable quit rates. Therefore, patients can be given the choice to quit in either of these ways (Chap. 470).
Inherited Predisposition to Lung Cancer Exposure to environmental carcinogens, such as those found in tobacco smoke, induce or facilitate the transformation from bronchoepithelial cells to the malignant phenotype. The contribution of carcinogens on transformation is modulated by polymorphic variations in genes that affect aspects of carcinogen metabolism. Certain genetic polymorphisms of the P450 enzyme system, specifically CYP1A1, and chromosome fragility are associated with the development of lung cancer. These genetic variations occur at relatively high frequency in the population, but their contribution to an individual’s lung cancer risk is generally low. However, because of their population frequency, the overall impact on lung cancer risk could be high. In addition, environmental factors, as modified by inherited modulators, likely affect specific genes by deregulating important pathways to enable the cancer phenotype.
First-degree relatives of lung cancer probands have a two- to threefold excess risk of lung cancer and other cancers, many of which are not smoking-related. These data suggest that specific genes and/or genetic variants may contribute to susceptibility to lung cancer. However, very few such genes have yet been identified. Individuals with inherited mutations in RB (patients with retinoblastoma living to adulthood) and p53 (Li-Fraumeni syndrome) genes may develop lung cancer. Common gene variants involved in lung cancer have been recently identified through large, collaborative, genome-wide association studies. These studies identified three separate loci that are associated with lung cancer (5p15, 6p21, and 15q25) and include genes that regulate acetylcholine nicotinic receptors and telomerase production. A rare germline mutation (T790M) involving the epidermal growth factor receptor (EGFR) maybe be linked to lung cancer susceptibility in never smokers. Likewise, a susceptibility locus on chromosome 6q greatly increases risk lung cancer risk among light and never smokers. Although progress has been made, there is a significant amount of work that remains to be done in identifying heritable risk factors for lung cancer. Currently no molecular criteria are suitable to select patients for more intense screening programs or for specific chemopreventative strategies.
PATHOLOGY
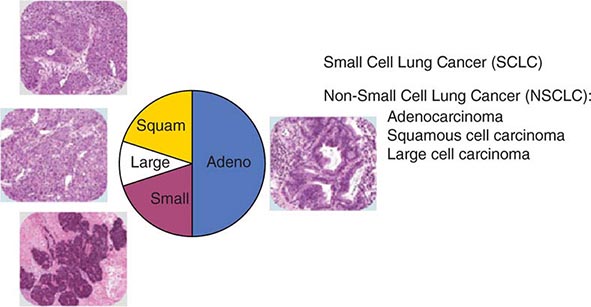
The World Health Organization (WHO) defines lung cancer as tumors arising from the respiratory epithelium (bronchi, bronchioles, and alveoli). The WHO classification system divides epithelial lung cancers into four major cell types: small-cell lung cancer (SCLC), adenocarcinoma, squamous cell carcinoma, and large-cell carcinoma; the latter three types are collectively known as non-small-cell carcinomas (NSCLCs) (Fig. 107-1). Small-cell carcinomas consist of small cells with scant cytoplasm, ill-defined cell borders, finely granular nuclear chromatin, absent or inconspicuous nucleoli, and a high mitotic count. SCLC may be distinguished from NSCLC by the presence of neuroendocrine markers including CD56, neural cell adhesion molecule (NCAM), synaptophysin, and chromogranin. In North America, adenocarcinoma is the most common histologic type of lung cancer. Adenocarcinomas possess glandular differentiation or mucin production and may show acinar, papillary, lepidic, or solid features or a mixture of these patterns. Squamous cell carcinomas of the lung are morphologically identical to extrapulmonary squamous cell carcinomas and cannot be distinguished by immunohistochemistry alone. Squamous cell tumors show keratinization and/or intercellular bridges that arise from bronchial epithelium. The tumor tends to consists of sheets of cells rather than the three-dimensional groups of cells characteristic of adenocarcinomas. Large-cell carcinomas comprise less than 10% of lung carcinomas. These tumors lack the cytologic and architectural features of small-cell carcinoma and glandular or squamous differentiation. Together these four histologic types account for approximately 90% of all epithelial lung cancers.
FIGURE 107-1 Traditional histologic view of lung cancer.
All histologic types of lung cancer can develop in current and former smokers, although squamous and small-cell carcinomas are most commonly associated with heavy tobacco use. Through the first half of the twentieth century, squamous carcinoma was the most common subtype of NSCLC diagnosed in the United States. However, with the decline in cigarette consumption over the past four decades, adenocarcinoma has become the most frequent histologic subtype of lung cancer in the United States as both squamous carcinoma and small-cell carcinoma are on the decline. In lifetime never smokers or former light smokers (<10 pack-year history), women, and younger adults (<60 years), adenocarcinoma tends to be the most common form of lung cancer.
Historically, the major pathologic distinction was simply between SCLC and NSCLC, because these tumors have quite different natural histories and therapeutic approaches (see below). Likewise, until fairly recently, there was no apparent need to distinguish among the various subtypes of NSCLC because there were no clear differences in therapeutic outcome based on histology alone. However, this perspective radically changed in 2004 with the recognition that a small percentage of lung adenocarcinomas harbored mutation in EGFR that rendered those tumors exquisitely sensitive to inhibitors of the EGFR tyrosine kinases (e.g., gefitinib and erlotinib). This observation, coupled with the subsequent identification of other “actionable” molecular alterations (Table 107-1) and the recognition that some active chemotherapy agents performed quite differently in squamous carcinomas versus adenocarcinomas, firmly established the need for modifications in the then-existing 2004 WHO lung cancer classification system. The revised 2011 classification system, developed jointly by the International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society, provides an integrated approach to the classification of lung adenocarcinomas that includes clinical, molecular, radiographic, and pathologic information. It also recognizes that most lung cancers present in an advanced stage and are often diagnosed based on small biopsies or cytologic specimens, rendering clear histologic distinctions difficult if not impossible.
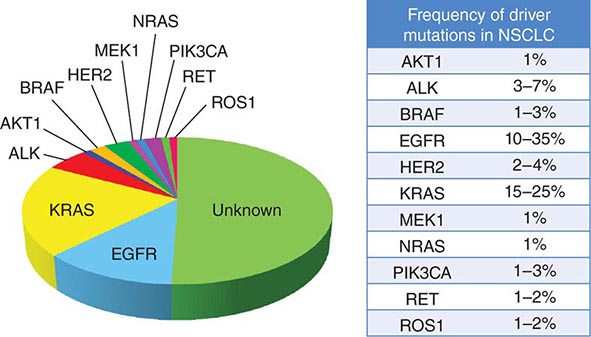
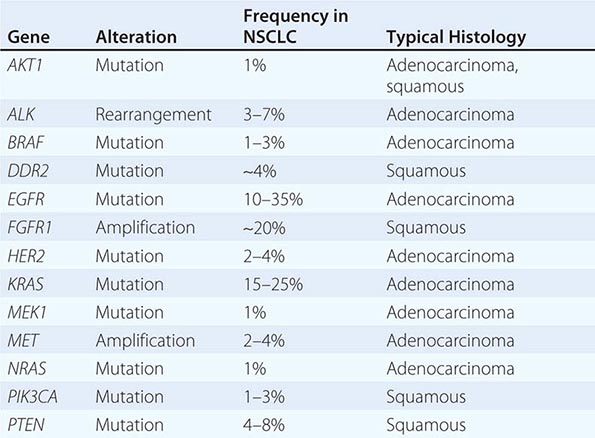
DRIVER MUTATIONS IN NON-SMALL-CELL LUNG CANCER (NSCLC) |
Previously, in the 2004 classification system, tumors failing to show definite glandular or squamous morphology in a small biopsy or cytologic specimen were simply classified as non-small-cell carcinoma, not otherwise specified. However, because the distinction between adenocarcinoma and squamous carcinoma is now viewed as critical to optimal therapeutic decision making, the modified classification approach recommends these lesions be further characterized using a limited special stain workup. This distinction can be achieved using a single marker for adenocarcinoma (thyroid transcription factor-1 or napsin-A) plus a squamous marker (p40 or p63) and/or mucin stains. The modified classification system also recommends preservation of sufficient specimen material for appropriate molecular testing necessary to help guide therapeutic decision making (see below).
Another significant modification to the WHO classification system is the discontinuation of the terms bronchioloalveolar carcinoma and mixed-subtype adenocarcinoma. The term bronchioloalveolar carcinoma was dropped due to its inconsistent use and because it caused confusion in routine clinical care and research. As formerly used, the term encompassed at least five different entities with diverse clinical and molecular properties. The terms adenocarcinoma in situ and minimally invasive adenocarcinoma are now recommended for small solitary adenocarcinomas (≤3 cm) with either pure lepidic growth (term used to describe single-layered growth of atypical cuboidal cells coating the alveolar walls) or predominant lepidic growth with ≤5 mm invasion. Individuals with these entities experience 100% or near 100% 5-year disease-free survival with complete tumor resection. Invasive adenocarcinomas, representing more than 70–90% of surgically resected lung adenocarcinomas, are now classified by their predominant pattern: lepidic, acinar, papillary, and solid patterns. Lepidic-predominant subtype has a favorable prognosis, acinar and papillary have an intermediate prognosis, and solid-predominant has a poor prognosis. The terms signet ring and clear cell adenocarcinoma have been eliminated from the variants of invasive lung adenocarcinoma, whereas the term micropapillary, a subtype with a particularly poor prognosis, has been added. Although EGFR mutations are encountered most frequently in nonmucinous adenocarcinomas with a lepidic- or papillary-predominant pattern, most adenocarcinoma subtypes can harbor EGFR or KRAS mutations. The same is true of ALK, RET, and ROS1 rearrangements. What was previously termed mucinous bronchioloalveolar carcinoma is now called invasive mucinous adenocarcinoma. These tumors generally lack EGFR mutations and show a strong correlation with KRAS mutations. Overall, the revised WHO reclassification of lung cancer addresses important advances in diagnosis and treatment, most importantly, the critical advances in understanding the specific genes and molecular pathways that initiate and sustain lung tumorigenesis resulting in new “targeted” therapies with improved specificity and better antitumor efficacy.
IMMUNOHISTOCHEMISTRY
The diagnosis of lung cancer most often rests on the morphologic or cytologic features correlated with clinical and radiographic findings. Immunohistochemistry may be used to verify neuroendocrine differentiation within a tumor, with markers such as neuron-specific enolase (NSE), CD56 or NCAM, synaptophysin, chromogranin, and Leu7. Immunohistochemistry is also helpful in differentiating primary from metastatic adenocarcinomas; thyroid transcription factor-1 (TTF-1), identified in tumors of thyroid and pulmonary origin, is positive in over 70% of pulmonary adenocarcinomas and is a reliable indicator of primary lung cancer, provided a thyroid primary has been excluded. A negative TTF-1, however, does not exclude the possibility of a lung primary. TTF-1 is also positive in neuroendocrine tumors of pulmonary and extrapulmonary origin. Napsin-A (Nap-A) is an aspartic protease that plays an important role in maturation of surfactant B7 and is expressed in cytoplasm of type II pneumocytes. In several studies, Nap-A has been reported in >90% of primary lung adenocarcinomas. Notably, a combination of Nap-A and TTF-1 is useful in distinguishing primary lung adenocarcinoma (Nap-A positive, TTF-1 positive) from primary lung squamous cell carcinoma (Nap-A negative, TTF-1 negative) and primary SCLC (Nap-A negative, TTF-1 positive). Cytokeratins 7 and 20 used in combination can help narrow the differential diagnosis; nonsquamous NSCLC, SCLC, and mesothelioma may stain positive for CK7 and negative for CK20, whereas squamous cell lung cancer often will be both CK7 and CK20 negative. p63 is a useful marker for the detection of NSCLCs with squamous differentiation when used in cytologic pulmonary samples. Mesothelioma can be easily identified ultrastructurally, but it has historically been difficult to differentiate from adenocarcinoma through morphology and immunohistochemical staining. Several markers in the last few years have proven to be more helpful including CK5/6, calretinin, and Wilms tumor gene-1 (WT-1), all of which show positivity in mesothelioma.
MOLECULAR PATHOGENESIS
Cancer is a disease involving dynamic changes in the genome. As proposed by Hanahan and Weinberg, virtually all cancer cells acquire six hallmark capabilities: self-sufficiency in growth signals, insensitivity to antigrowth signals, evading apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis. The order in which these hallmark capabilities are acquired appears quite variable and can differ from tumor to tumor. Events leading to acquisition of these hallmarks can vary widely, although broadly, cancers arise as a result from accumulations of gain-of-function mutations in oncogenes and loss-of-function mutations in tumor-suppressor genes. Further complicating the study of lung cancer, the sequence of events that lead to disease is clearly different for the various histopathologic entities.
The exact cell of origin for lung cancers is not clearly defined. Whether one cell of origin leads to all histologic forms of lung cancer is unclear. However, for lung adenocarcinoma, evidence suggests that type II epithelial cells (or alveolar epithelial cells) have the capacity to give rise to tumors. For SCLC, cells of neuroendocrine origin have been implicated as precursors.
For cancers in general, one theory holds that a small subset of the cells within a tumor (i.e., “stem cells”) are responsible for the full malignant behavior of the tumor. As part of this concept, the large bulk of the cells in a cancer are “offspring” of these cancer stem cells. While clonally related to the cancer stem cell subpopulation, most cells by themselves cannot regenerate the full malignant phenotype. The stem cell concept may explain the failure of standard medical therapies to eradicate lung cancers, even when there is a clinical complete response. Disease recurs because therapies do not eliminate the stem cell component, which may be more resistant to chemotherapy. Precise human lung cancer stem cells have yet to be identified.
Lung cancer cells harbor multiple chromosomal abnormalities, including mutations, amplifications, insertions, deletions, and translocations. One of the earliest sets of oncogenes found to be aberrant was the MYC family of transcription factors (MYC, MYCN, and MYCL). MYC is most frequently activated via gene amplification or transcriptional dysregulation in both SCLC and NSCLC. Currently, there are no MYC-specific drugs.
Among lung cancer histologies, adenocarcinomas have been the most extensively catalogued for recurrent genomic gains and losses as well as for somatic mutations (Fig. 107-2). While multiple different kinds of aberrations have been found, a major class involves “driver mutations,” which are mutations that occur in genes encoding signaling proteins that when aberrant, drive initiation and maintenance of tumor cells. Importantly, driver mutations can serve as potential Achilles’ heels for tumors, if their gene products can be targeted appropriately. For example, one set of mutations involves EGFR, which belongs to the ERBB (HER) family of protooncogenes, including EGFR (ERBB1), HER2/neu (ERBB2), HER3 (ERBB3), and HER4 (ERBB4). These genes encode cell-surface receptors consisting of an extracellular ligand-binding domain, a transmembrane structure, and an intracellular tyrosine kinase (TK) domain. The binding of ligand to receptor activates receptor dimerization and TK autophosphorylation, initiating a cascade of intracellular events, and leading to increased cell proliferation, angiogenesis, metastasis, and a decrease in apoptosis. Lung adenocarcinomas can arise when tumors express mutant EGFR. These same tumors display high sensitivity to small-molecule EGFR TK inhibitors (TKIs). Additional examples of driver mutations in lung adenocarcinoma include the GTPase KRAS, the serine-threonine kinase BRAF, and the lipid kinase PIK3CA. More recently, more subsets of lung adenocarcinoma have been identifed as defined by the presence of specific chromsomal rearrangements resulting in the abberant activation of the TKs ALK, ROS1, and RET. Notably, most driver mutations in lung cancer appear to be mutually exclusive, suggesting that acquisition of one of these mutations is sufficient to drive tumorigenesis. Although driver mutations have mostly been found in adenocarinomas, three potential molecular targets recently have been identified in squamous cell lung carcinomas: FGFR1 amplification, DDR2 mutations, and PIK3CA mutations/PTEN loss (Table 107-1). Together, these potentially “actionable” defects occur in up to 50% of squamous carcinomas.
FIGURE 107-2 Driver mutations in adenocarcinomas.
A large number of tumor-suppressor genes have also been identified that are inactivated during the pathogenesis of lung cancer. These include TP53, RB1, RASSF1A, CDKN2A/B, LKB1 (STK11), and FHIT. Nearly 90% of SCLCs harbor mutations in TP53 and RB1. Several tumor-suppressor genes on chromosome 3p appear to be involved in nearly all lung cancers. Allelic loss for this region occurs very early in lung cancer pathogenesis, including in histologically normal smoking-damaged lung epithelium.
EARLY DETECTION AND SCREENING
In lung cancer, clinical outcome is related to the stage at diagnosis, and hence, it is generally assumed that early detection of occult tumors will lead to improved survival. Early detection is a process that involves screening tests, surveillance, diagnosis, and early treatment. Screening refers to the use of simple tests across a healthy population in order to identify individuals who harbor asymptomatic disease. For a screening program to be successful, there must be a high burden of disease within the target population; the test must be sensitive, specific, accessible, and cost effective; and there must be effective treatment that can reduce mortality. With any screening procedure, it is important to consider the possible influence of lead-time bias (detecting the cancer earlier without an effect on survival), length-time bias (indolent cancers are detected on screening and may not affect survival, whereas aggressive cancers are likely to cause symptoms earlier in patients and are less likely to be detected), and overdiagnosis (diagnosing cancers so slow growing that they are unlikely to cause the death of the patient) (Chap. 100).
Because a majority of lung cancer patients present with advanced disease beyond the scope of surgical resection, there is understandable skepticism about the value of screening in this condition. Indeed, randomized controlled trials conducted in the 1960s to 1980s using screening chest x-rays (CXR), with or without sputum cytology, reported no impact on lung cancer–specific mortality in patients characterized as high risk (males age ≥45 years with a smoking history). These studies have been criticized for their design, statistical analyses, and outdated imaging modalities. The results of the more recently conducted Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) are consistent with these earlier reports. Initiated in 1993, participants in the PLCO lung cancer screening trial received annual CXR screening for 4 years, whereas participants in the usual care group received no interventions other than their customary medical care. The diagnostic follow-up of positive screening results was determined by participants and their physicians. The PLCO trial differed from previous lung cancer screening studies in that women and never smokers were eligible. The study was designed to detect a 10% reduction in lung cancer mortality in the interventional group. A total of 154,901 individuals between 55 and 74 years of age were enrolled (77,445 assigned to annual CXR screenings; 77,456 assigned to usual care). Participant demographics and tumor characteristics were well balanced between the two groups. Through 13 years of follow-up, cumulative lung cancer incidence rates (20.1 vs 19.2 per 10,000 person-years; rate ratio [RR], 1.05; 95% confidence interval [CI], 0.98–1.12) and lung cancer mortality (n = 1213 vs n = 1230) were identical between the two groups. The stage and histology of detected cancers in the two groups also were similar. These data corroborate previous recommendations against CXR screening for lung cancer.
In contrast to CXR, low-dose, noncontrast, thin-slice spiral chest computed tomography (LDCT) has emerged as an effective tool to screen for lung cancer. In nonrandomized studies conducted in the 1990s, LDCT scans were shown to detect more lung nodules and cancers than standard CXR in selected high-risk populations (e.g., age ≥60 years and a smoking history of ≥10 pack-years). Notably, up to 85% of the lung cancers discovered in these trials were classified as stage I disease and therefore considered potentially curable with surgical resection.
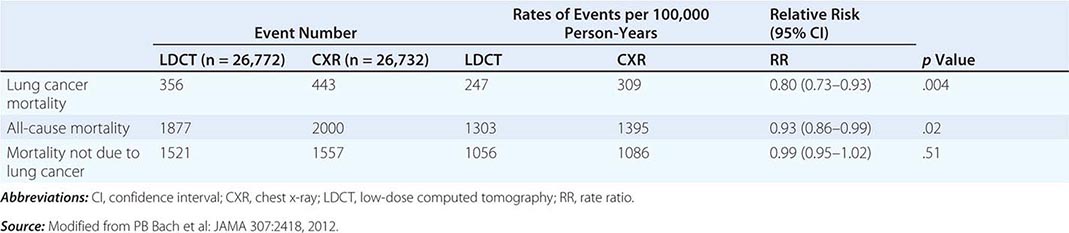
These data prompted the National Cancer Institute (NCI) to initiate the National Lung Screening Trial (NLST), a randomized study designed to determine if LDCT screening could reduce mortality from lung cancer in high-risk populations as compared with standard posterior anterior CXR. High-risk patients were defined as individuals between 55 and 74 years of age, with a ≥30 pack-year history of cigarette smoking; former smokers must have quit within the previous 15 years. Excluded from the trial were individuals with a previous lung cancer diagnosis, a history of hemoptysis, an unexplained weight loss of >15 lb in the preceding year, or a chest CT within 18 months of enrollment. A total of 53,454 persons were enrolled and randomized to annual screening yearly for three years (LDCT screening, n = 26,722; CXR screening, n = 26,732). Any noncalcified nodule measuring ≥4 mm in any diameter found on LDCT and CXR images with any noncalcified nodule or mass were classified as “positive.” Participating radiologists had the option of not calling a final screen positive if a noncalcified nodule had been stable on the three screening exams. Overall, 39.1% of participants in the LDCT group and 16% in the CXR group had at least one positive screening result. Of those who screened positive, the false-positive rate was 96.4% in the LDCT group and 94.5% in the CXR group. This was consistent across all three rounds. In the LDCT group, 1060 cancers were identified compared with 941 cancers in the CXR group (645 vs 572 per 100,000 person-years; RR, 1.13; 95% CI, 1.03 to 1.23). Nearly twice as many early-stage IA cancers were detected in the LDCT group compared with the CXR group (40% vs 21%). The overall rates of lung cancer death were 247 and 309 deaths per 100,000 participants in the LDCT and CXR groups, respectively, representing a 20% reduction in lung cancer mortality in the LDCT-screened population (95% CI, 6.8–26.7%; p = .004). Compared with the CXR group, the rate of death in the LDCT group from any cause was reduced by 6.7% (95% CI, 1.2–13.6; p = .02) (Table 107-2). The number needed to screen (NNTS) to prevent one lung cancer death was calculated to be 320.
RESULTS OF NATIONAL LUNG SCREENING TRIAL |
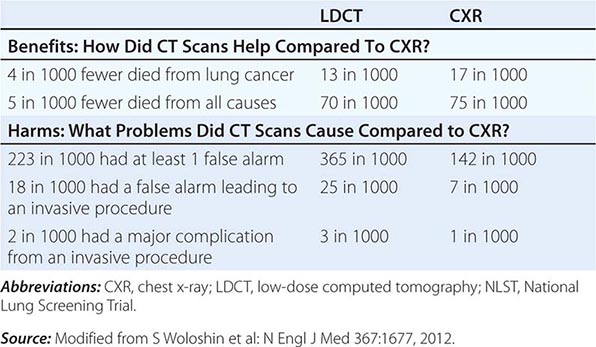
LDCT screening for lung cancer comes with known risks including a high rate of false-positive results, false-negative results, potential for unnecessary follow-up testing, radiation exposure, overdiagnosis, changes in anxiety and quality of life, and substantial financial costs. By far the biggest challenge confronting the use of CT screening is the high false-positive rate. False positives can have a substantial impact on patients through the expense and risk of unneeded further evaluation and emotional stress. The management of these patients usually consists of serial CT scans over time to see if the nodules grow, attempted fine-needle aspirates, or surgical resection. At $300 per scan (NCI estimated cost), the outlay for initial LDCT alone could run into the billions of dollars annually, an expense that only further escalates when factoring in various downstream expenditures an individual might incur in the assessment of positive findings. A formal cost-effectiveness analysis of the NLST is expected soon that should help resolve this crucial concern.
Despite the aforementioned caveats, screening of individuals who meet the NLST criteria for lung cancer risk (or in some cases, modified versions of these criteria) seems warranted, provided comprehensive multidisciplinary coordinated care and follow-up similar to those provided to NLST participants are available. Algorithms to improve candidate selection are under development. When discussing the option of LDCT screening, use of absolute risks rather than relative risks is helpful because studies indicate the public can process absolute terminology more effectively than relative risk projections. A useful guide has been developed by the NCI to help patients and physicians assess the benefits and harms of LDCT screening for lung cancer (Table 107-3). Finally, even a small negative effect of screening on smoking behavior (either lower quit rates or higher recidivism) could easily offset the potential gains in a population. Fortunately no such impact has been reported to date. Nonetheless, smoking cessation must be included as an indispensable component of any screening program.
THE BENEFITS AND HARMS OF LDCT SCREENING FOR LUNG CANCER BASED ON NLST DATA |
CLINICAL MANIFESTATIONS
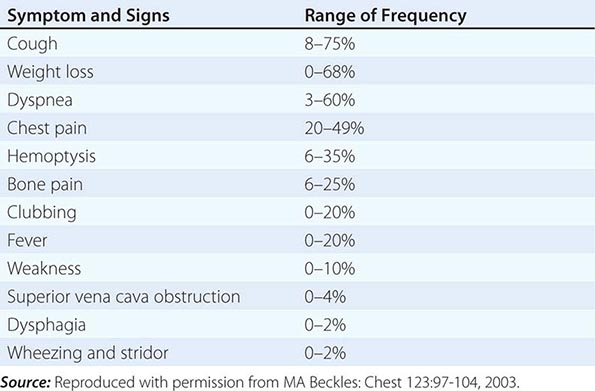
Over half of all patients diagnosed with lung cancer present with locally advanced or metastatic disease at the time of diagnosis. The majority of patients present with signs, symptoms, or laboratory abnormalities that can be attributed to the primary lesion, local tumor growth, invasion or obstruction of adjacent structures, growth at distant metastatic sites, or a paraneoplastic syndrome (Tables 107-4 and 107-5). The prototypical lung cancer patient is a current or former smoker of either sex, usually in the seventh decade of life. A history of chronic cough with or without hemoptysis in a current or former smoker with chronic obstructive pulmonary disease (COPD) age 40 years or older should prompt a thorough investigation for lung cancer even in the face of a normal CXR. A persistent pneumonia without constitutional symptoms and unresponsive to repeated courses of antibiotics also should prompt an evaluation for the underlying cause. Lung cancer arising in a life-time never smoker is more common in women and East Asians. Such patients also tend to be younger than their smoking counterparts at the time of diagnosis. The clinical presentation of lung cancer in never smokers tends to mirror that of current and former smokers.
PRESENTING SIGNS AND SYMPTOMS OF LUNG CANCER |
CLINICAL FINDINGS SUGGESTIVE OF METASTATIC DISEASE |
Patients with central or endobronchial growth of the primary tumor may present with cough, hemoptysis, wheeze, stridor, dyspnea, or postobstructive pneumonitis. Peripheral growth of the primary tumor may cause pain from pleural or chest wall involvement, dyspnea on a restrictive basis, and symptoms of a lung abscess resulting from tumor cavitation. Regional spread of tumor in the thorax (by contiguous growth or by metastasis to regional lymph nodes) may cause tracheal obstruction, esophageal compression with dysphagia, recurrent laryngeal paralysis with hoarseness, phrenic nerve palsy with elevation of the hemidiaphragm and dyspnea, and sympathetic nerve paralysis with Horner’s syndrome (enophthalmos, ptosis, miosis, and anhydrosis). Malignant pleural effusions can cause pain, dyspnea, or cough. Pancoast (or superior sulcus tumor) syndromes result from local extension of a tumor growing in the apex of the lung with involvement of the eighth cervical and first and second thoracic nerves, and present with shoulder pain that characteristically radiates in the ulnar distribution of the arm, often with radiologic destruction of the first and second ribs. Often Horner’s syndrome and Pancoast syndrome coexist. Other problems of regional spread include superior vena cava syndrome from vascular obstruction; pericardial and cardiac extension with resultant tamponade, arrhythmia, or cardiac failure; lymphatic obstruction with resultant pleural effusion; and lymphangitic spread through the lungs with hypoxemia and dyspnea. In addition, lung cancer can spread transbronchially, producing tumor growth along multiple alveolar surfaces with impairment of gas exchange, respiratory insufficiency, dyspnea, hypoxemia, and sputum production. Constitutional symptoms may include anorexia, weight loss, weakness, fever, and night sweats. Apart from the brevity of symptom duration, these parameters fail to clearly distinguish SCLC from NSCLC or even from neoplasms metastatic to lungs.
Extrathoracic metastatic disease is found at autopsy in more than 50% of patients with squamous carcinoma, 80% of patients with adenocarcinoma and large-cell carcinoma, and greater than 95% of patients with SCLC. Approximately one-third of patients present with symptoms as a result of distant metastases. Lung cancer metastases may occur in virtually every organ system, and the site of metastatic involvement largely determines other symptoms. Patients with brain metastases may present with headache, nausea and vomiting, seizures, or neurologic deficits. Patients with bone metastases may present with pain, pathologic fractures, or cord compression. The latter may also occur with epidural metastases. Individuals with bone marrow invasion may present with cytopenias or leukoerythroblastosis. Those with liver metastases may present with hepatomegaly, right upper quadrant pain, fever, anorexia, and weight loss. Liver dysfunction and biliary obstructions are rare. Adrenal metastases are common but rarely cause pain or adrenal insufficiency unless they are large.
Paraneoplastic syndromes are common in patients with lung cancer, especially those with SCLC, and may be the presenting finding or the first sign of recurrence. In addition, paraneoplastic syndromes may mimic metastatic disease and, unless detected, lead to inappropriate palliative rather than curative treatment. Often the paraneoplastic syndrome may be relieved with successful treatment of the tumor. In some cases, the pathophysiology of the paraneoplastic syndrome is known, particularly when a hormone with biological activity is secreted by a tumor. However, in many cases, the pathophysiology is unknown. Systemic symptoms of anorexia, cachexia, weight loss (seen in 30% of patients), fever, and suppressed immunity are paraneoplastic syndromes of unknown etiology or at least not well defined. Weight loss greater than 10% of total body weight is considered a bad prognostic sign. Endocrine syndromes are seen in 12% of patients; hypercalcemia resulting from ectopic production of parathyroid hormone (PTH), or more commonly, PTH-related peptide, is the most common life-threatening metabolic complication of malignancy, primarily occurring with squamous cell carcinomas of the lung. Clinical symptoms include nausea, vomiting, abdominal pain, constipation, polyuria, thirst, and altered mental status.
Hyponatremia may be caused by the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) or possibly atrial natriuretic peptide (ANP). SIADH resolves within 1-4 weeks of initiating chemotherapy in the vast majority of cases. During this period, serum sodium can usually be managed and maintained above 128 mEq/L via fluid restriction. Demeclocycline can be a useful adjunctive measure when fluid restriction alone is insufficient. Vasopressin receptor antagonists like tolvaptan also have been used in the management of SIADH. However, there are significant limitations to the use of tolvaptan including liver injury and overly rapid correction of the hyponatremia, which can lead to irreversible neurologic injury. Likewise, the cost of tolvaptan may be prohibitive (as high as $300 per tablet in some areas). Of note, patients with ectopic ANP may have worsening hyponatremia if sodium intake is not concomitantly increased. Accordingly, if hyponatremia fails to improve or worsens after 3–4 days of adequate fluid restriction, plasma levels of ANP should be measured to determine the causative syndrome.
Ectopic secretion of ACTH by SCLC and pulmonary carcinoids usually results in additional electrolyte disturbances, especially hypokalemia, rather than the changes in body habitus that occur in Cushing’s syndrome from a pituitary adenoma. Treatment with standard medications, such as metyrapone and ketoconazole, is largely ineffective due to extremely high cortisol levels. The most effective strategy for management of the Cushing’s syndrome is effective treatment of the underlying SCLC. Bilateral adrenalectomy may be considered in extreme cases.
Skeletal–connective tissue syndromes include clubbing in 30% of cases (usually NSCLCs) and hypertrophic primary osteoarthropathy in 1–10% of cases (usually adenocarcinomas). Patients may develop periostitis, causing pain, tenderness, and swelling over the affected bones and a positive bone scan. Neurologic-myopathic syndromes are seen in only 1% of patients but are dramatic and include the myasthenic Eaton-Lambert syndrome and retinal blindness with SCLC, whereas peripheral neuropathies, subacute cerebellar degeneration, cortical degeneration, and polymyositis are seen with all lung cancer types. Many of these are caused by autoimmune responses such as the development of anti-voltage-gated calcium channel antibodies in Eaton-Lambert syndrome. Patients with this disorder present with proximal muscle weakness, usually in the lower extremities, occasional autonomic dysfunction, and rarely, cranial nerve symptoms or involvement of the bulbar or respiratory muscles. Depressed deep tendon reflexes are frequently present. In contrast to patients with myasthenia gravis, strength improves with serial effort. Some patients who respond to chemotherapy will have resolution of the neurologic abnormalities. Thus, chemotherapy is the initial treatment of choice. Paraneoplastic encephalomyelitis and sensory neuropathies, cerebellar degeneration, limbic encephalitis, and brainstem encephalitis occur in SCLC in association with a variety of antineuronal antibodies such as anti-Hu, anti-CRMP5, and ANNA-3. Paraneoplastic cerebellar degeneration may be associated with anti-Hu, anti-Yo, or P/Q calcium channel autoantibodies. Coagulation or thrombotic or other hematologic manifestations occur in 1–8% of patients and include migratory venous thrombophlebitis (Trousseau’s syndrome), nonbacterial thrombotic (marantic) endocarditis with arterial emboli, and disseminated intravascular coagulation with hemorrhage, anemia, granulocytosis, and leukoerythroblastosis. Thrombotic disease complicating cancer is usually a poor prognostic sign. Cutaneous manifestations such as dermatomyositis and acanthosis nigricans are uncommon (1%), as are the renal manifestations of nephrotic syndrome and glomerulonephritis (≤1%).
DIAGNOSING LUNG CANCER
Tissue sampling is required to confirm a diagnosis in all patients with suspected lung cancer. In patients with suspected metastatic disease, a biopsy of the most distant site of disease is preferred for tissue confirmation. Given the greater emphasis placed on molecular testing for NSCLC patients, a core biopsy is preferred to ensure adequate tissue for analysis. Tumor tissue may be obtained via minimally invasive techniques such as bronchial or transbronchial biopsy during fiberoptic bronchoscopy, by fine-needle aspiration or percutaneous biopsy using image guidance, or via endobronchial ultrasound (EBUS) guided biopsy. Depending on the location, lymph node sampling may occur via transesophageal endoscopic ultrasound-guided biopsy (EUS), EBUS, or blind biopsy. In patients with clinically palpable disease such as a lymph node or skin metastasis, a biopsy may be obtained. In patients with suspected metastatic disease, a diagnosis may be confirmed by percutaneous biopsy of a soft tissue mass, lytic bone lesion, bone marrow, pleural or liver lesion, or an adequate cell block obtained from a malignant pleural effusion. In patients with a suspected malignant pleural effusion, if the initial thoracentesis is negative, a repeat thoracentesis is warranted. Although the majority of pleural effusions are due to malignant disease, particularly if they are exudative or bloody, some may be parapneumonic. In the absence of distant disease, such patients should be considered for possible curative treatment.
The diagnostic yield of any biopsy depends on several factors including location (accessibility) of the tumor, tumor size, tumor type, and technical aspects of the diagnostic procedure including the experience level of the bronchoscopist and pathologist. In general, central lesions such as squamous cell carcinomas, small-cell carcinomas, or endobronchial lesions such as carcinoid tumors are more readily diagnosed by bronchoscopic examination, whereas peripheral lesions such as adenocarcinomas and large-cell carcinomas are more amenable to transthoracic biopsy. Diagnostic accuracy for SCLC versus NSCLC for most specimens is excellent, with lesser accuracy for subtypes of NSCLC.
Bronchoscopic specimens include bronchial brush, bronchial wash, bronchioloalveolar lavage, transbronchial fine-needle aspiration (FNA), and core biopsy. For more accurate histologic classification, mutation analysis, or investigational purposes, reasonable efforts (e.g., a core needle biopsy) should be made to obtain more tissue than what is contained in a routine cytology specimen obtained by FNA. Overall sensitivity for combined use of bronchoscopic methods is approximately 80%, and together with tissue biopsy, the yield increases to 85–90%. Like transbronchial core biopsy specimens, transthoracic core biopsy specimens are also preferred. Sensitivity is highest for larger lesions and peripheral tumors. In general, core biopsy specimens, whether transbronchial, transthoracic, or EUS-guided, are superior to other specimen types. This is primarily due to the higher percentage of tumor cells with fewer confounding factors such as obscuring inflammation and reactive nonneoplastic cells.
Sputum cytology is inexpensive and noninvasive but has a lower yield than other specimen types due to poor preservation of the cells and more variability in acquiring a good-quality specimen. The yield for sputum cytology is highest for larger and centrally located tumors such as squamous cell carcinoma and small-cell carcinoma histology. The specificity for sputum cytology averages close to 100%, although sensitivity is generally <70%. The accuracy of sputum cytology improves with increased numbers of specimens analyzed. Consequently, analysis of at least three sputum specimens is recommended.
STAGING LUNG CANCER
Lung cancer staging consists of two parts: first, a determination of the location of the tumor and possible metastatic sites (anatomic staging), and second, an assessment of a patient’s ability to withstand various antitumor treatments (physiologic staging). All patients with lung cancer should have a complete history and physical examination, with evaluation of all other medical problems, determination of performance status, and history of weight loss. The most significant dividing line is between those patients who are candidates for surgical resection and those who are inoperable but will benefit from chemotherapy, radiation therapy, or both. Staging with regard to a patient’s potential for surgical resection is principally applicable to NSCLC.
ANATOMIC STAGING OF PATIENTS WITH LUNG CANCER
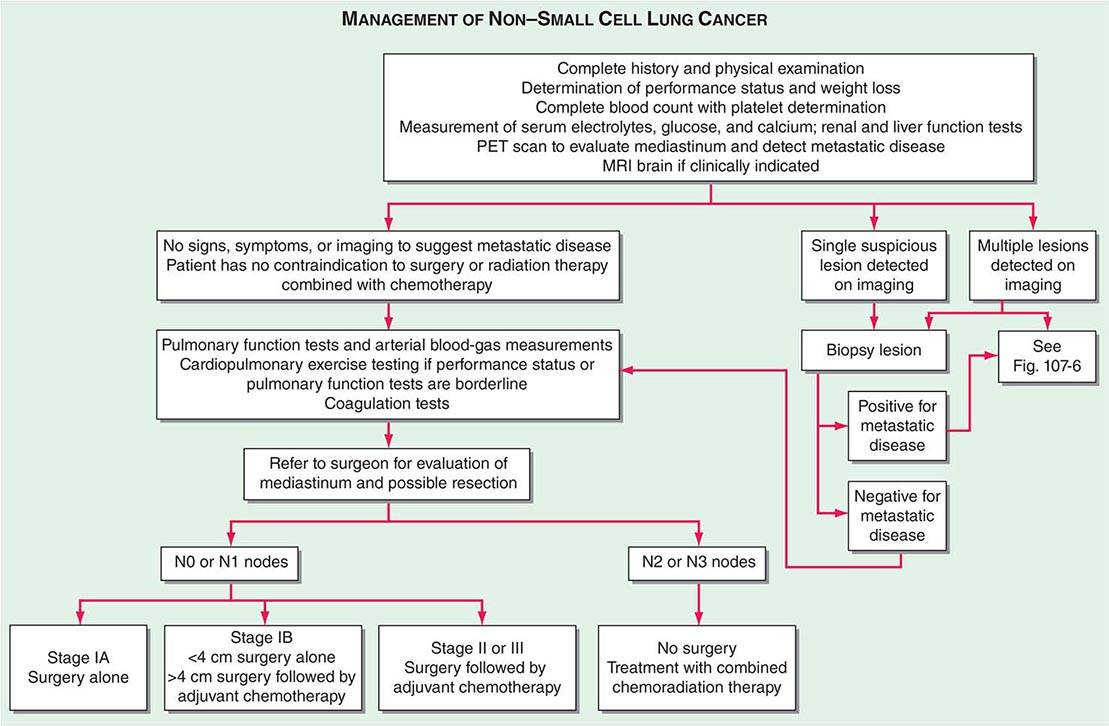
The accurate staging of patients with NSCLC is essential for determining the appropriate treatment in patients with resectable disease and avoiding unnecessary surgical procedures in patients with advanced disease (Fig. 107-3). All patients with NSCLC should undergo initial radiographic imaging with CT scan, positron emission tomography (PET), or preferably CT-PET. PET scanning attempts to identify sites of malignancy based on glucose metabolism by measuring the uptake of 18F-fluorodeoxyglucose (FDG). Rapidly dividing cells, presumably in the lung tumors, will preferentially take up 18F-FDG and appear as a “hot spot.” To date, PET has been mostly used for staging and detection of metastases in lung cancer and in the detection of nodules >15 mm in diameter. Combined 18F-FDG PET-CT imaging has been shown to improve the accuracy of staging in NSCLC compared to visual correlation of PET and CT or either study alone. CT-PET has been found to be superior in identifying pathologically enlarged mediastinal lymph nodes and extrathoracic metastases. A standardized uptake value (SUV) of >2.5 on PET is highly suspicious for malignancy. False negatives can be seen in diabetes, in lesions <8 mm, and in slow-growing tumors (e.g., carcinoid tumors or well-differentiated adenocarcinoma). False positives can be seen in certain infections and granulomatous disease (e.g., tuberculosis). Thus, PET should never be used alone to diagnose lung cancer, mediastinal involvement, or metastases. Confirmation with tissue biopsy is required. For brain metastases, magnetic resonance imaging (MRI) is the most effective method. MRI can also be useful in selected circumstances, such as superior sulcus tumors to rule out brachial plexus involvement, but in general, MRI does not play a major role in NSCLC staging.
FIGURE 107-3 Algorithm for management of non-small-cell lung cancer. MRI, magnetic resonance imaging; PET, positron emission tomography.
In patients with NSCLC, the following are contraindications to potential curative resection: extrathoracic metastases, superior vena cava syndrome, vocal cord and, in most cases, phrenic nerve paralysis, malignant pleural effusion, cardiac tamponade, tumor within 2 cm of the carina (potentially curable with combined chemoradiotherapy), metastasis to the contralateral lung, metastases to supraclavicular lymph nodes, contralateral mediastinal node metastases (potentially curable with combined chemoradiotherapy), and involvement of the main pulmonary artery. In situations where it will make a difference in treatment, abnormal scan findings require tissue confirmation of malignancy so that patients are not precluded from having potentially curative therapy.
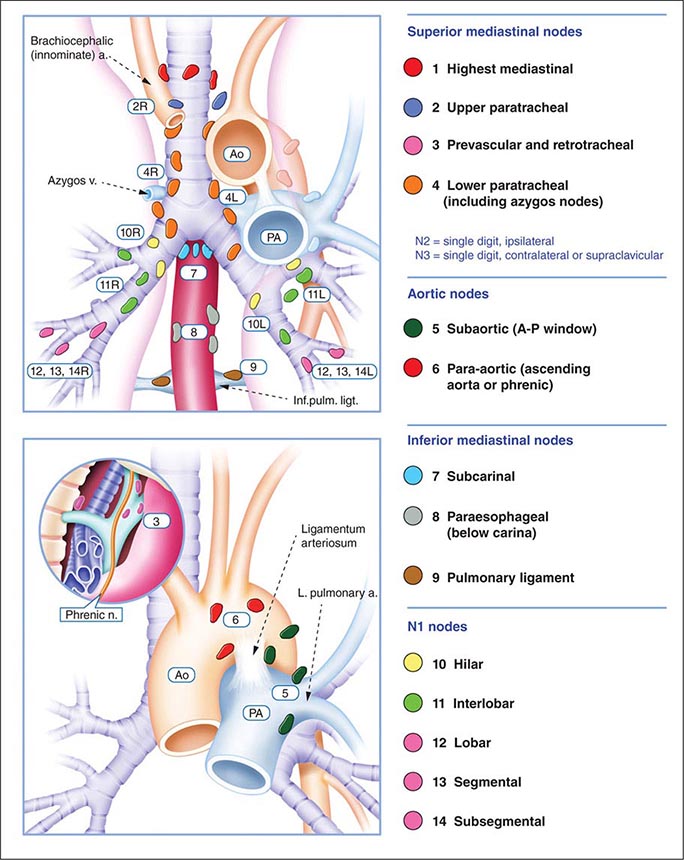
The best predictor of metastatic disease remains a careful history and physical examination. If signs, symptoms, or findings from the physical examination suggest the presence of malignancy, then sequential imaging starting with the most appropriate study should be performed. If the findings from the clinical evaluation are negative, then imaging studies beyond CT-PET are unnecessary and the search for metastatic disease is complete. More controversial is how one should assess patients with known stage III disease. Because these patients are more likely to have asymptomatic occult metastatic disease, current guidelines recommend a more extensive imaging evaluation including imaging of the brain with either CT scan or MRI. In patients in whom distant metastatic disease has been ruled out, lymph node status needs to be assessed via a combination of radiographic imaging and/or minimally invasive techniques such as those mentioned above and/or invasive techniques such as mediastinoscopy, mediastinotomy, thoracoscopy, or thoracotomy. Approximately one-quarter to one-half of patients diagnosed with NSCLC will have mediastinal lymph node metastases at the time of diagnosis. Lymph node sampling is recommended in all patients with enlarged nodes detected by CT or PET scan and in patients with large tumors or tumors occupying the inner third of the lung. The extent of mediastinal lymph node involvement is important in determining the appropriate treatment strategy: surgical resection followed by adjuvant chemotherapy versus combined chemoradiation alone (see below). A standard nomenclature for referring to the location of lymph nodes involved with lung cancer has evolved (Fig. 107-4).
FIGURE 107-4 Lymph node stations in staging non-small-cell lung cancer. The International Association for the Study of Lung Cancer (IASLC) lymph node map, including the proposed grouping of lymph node stations into “zones” for the purposes of prognostic analyses. a., artery; Ao, aorta; Inf. pulm. ligt., inferior pulmonary ligament; n., nerve; PA, pulmonary artery; v., vein.
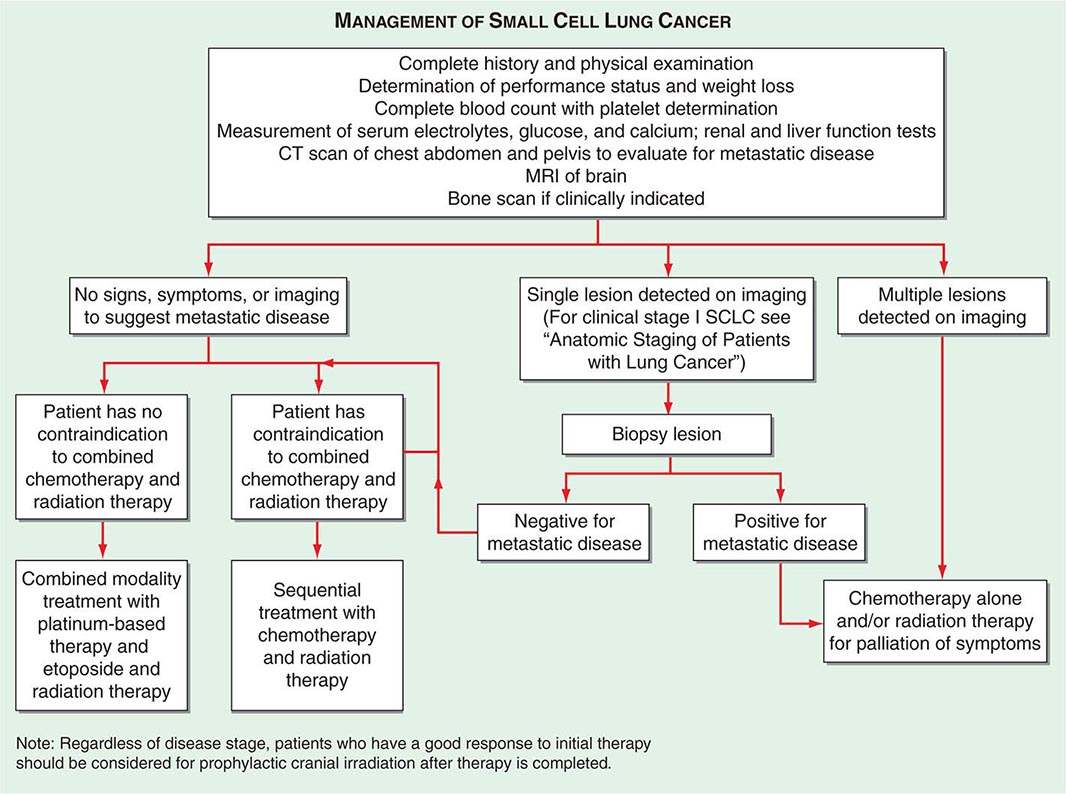
In SCLC patients, current staging recommendations include a CT scan of the chest and abdomen (because of the high frequency of hepatic and adrenal involvement), MRI of the brain (positive in 10% of asymptomatic patients), and radionuclide bone scan if symptoms or signs suggest disease involvement in these areas (Fig. 107-5). Although there are less data on the use of CT-PET in SCLC, the most recent American College of Chest Physicians Evidence-Based Clinical Practice Guidelines recommend PET scans in patients with clinical stage I SCLC who are being considered for curative intent surgical resection. In addition, invasive mediastinal staging and extrathoracic imaging (head MRI/CT and PET or abdominal CT plus bone scan) is also recommended for patients with clinical stage I SCLC if curative intent surgical resection is contemplated. Some practice guidelines also recommend the use of PET scanning in the staging of SCLC patients who are potential candidates for the addition of thoracic radiotherapy to chemotherapy. Bone marrow biopsies and aspirations are rarely performed now given the low incidence of isolated bone marrow metastases. Confirmation of metastatic disease, ipsilateral or contralateral lung nodules, or metastases beyond the mediastinum may be achieved by the same modalities recommended earlier for patients with NSCLC.
FIGURE 107-5 Algorithm for management of small-cell lung cancer. CT, computed tomography; MRI, magnetic resonance imaging.
If a patient has signs or symptoms of spinal cord compression (pain, weakness, paralysis, urinary retention), a spinal CT or MRI scan and examination of the cerebrospinal fluid cytology should be performed. If metastases are evident on imaging, a neurosurgeon should be consulted for possible palliative surgical resection and/or a radiation oncologist should be consulted for palliative radiotherapy to the site of compression. If signs or symptoms of leptomeningitis develop at any time in a patient with lung cancer, an MRI of the brain and spinal cord should be performed, as well as a spinal tap, for detection of malignant cells. If the spinal tap is negative, a repeat spinal tap should be considered. There is currently no approved therapy for the treatment of leptomeningeal disease.
STAGING SYSTEM FOR NON-SMALL-CELL LUNG CANCER
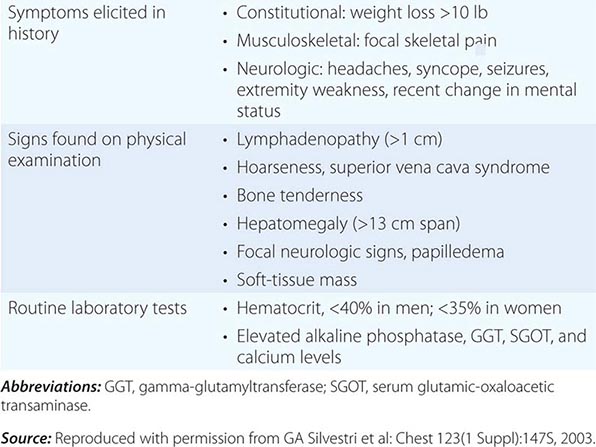
The tumor-node-metastasis (TNM) international staging system provides useful prognostic information and is used to stage all patients with NSCLC. The various T (tumor size), N (regional node involvement), and M (presence or absence of distant metastasis) are combined to form different stage groups (Tables 107-6 and 107-7). The previous edition of the TNM staging system for lung cancer was developed based on a relatively small database of patients from a single institution. The latest seventh edition of the TNM staging system went into effect in 2010 and developed using a much more robust database of more than 100,000 patients with lung cancer who were treated in multiple countries between 1990 and 2000. Data from 67,725 patients with NSCLC were then used to reevaluate the prognostic value of the TNM descriptors (Table 107-8). The major distinction between the sixth and seventh editions of the international staging systems is within the T classification; T1 tumors are divided into tumors ≤2 cm in size, as these patients were found to have a better prognosis compared to patients with tumors >2 cm but ≤3 cm. T2 tumors are divided into those that are >3 cm but ≤5 cm and those that are >5 cm but ≤7 cm. Tumors that are >7 cm are considered T3 tumors. T3 tumors also include tumors with invasion into local structures such as chest wall and diaphragm and additional nodules in the same lobe. T4 tumors include tumors of any size with invasion into mediastinum, heart, great vessels, trachea, or esophagus or multiple nodules in the ipsilateral lung. No changes have been made to the current classification of lymph node involvement (N). Patients with metastasis may be classified as M1a (malignant pleural or pericardial effusion, pleural nodules, or nodules in the contralateral lung) or M1b (distant metastasis; e.g., bone, liver, adrenal, or brain metastasis). Based on these data, approximately one-third of patients have localized disease that can be treated with curative attempt (surgery or radiotherapy), one-third have local or regional disease that may or may not be amenable to a curative attempt, and one-third have metastatic disease at the time of diagnosis.
SEVENTH EDITION TNM STAGING SYSTEMS FOR NON-SMALL-CELL LUNG CANCER |
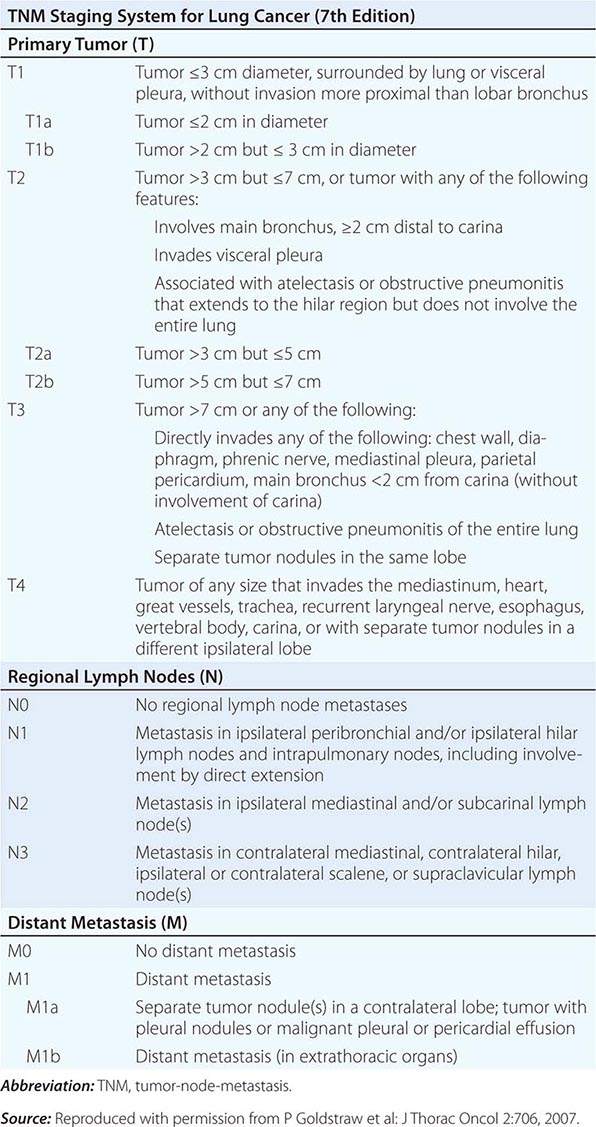
SEVENTH EDITION TNM STAGING SYSTEMS FOR NON-SMALL-CELL LUNG CANCER |
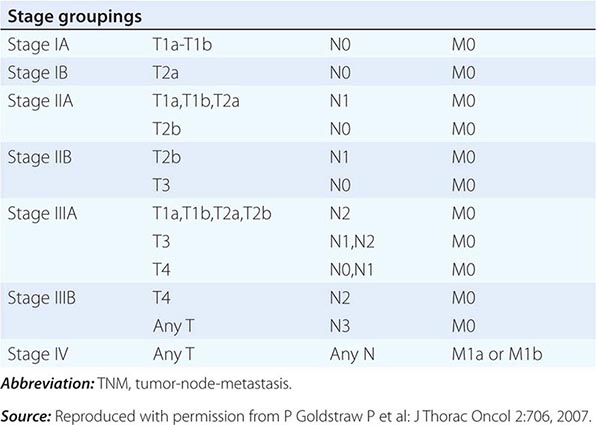
FIVE-YEAR SURVIVAL BY STAGE AND TNM CLASSIFICATION OF NON-SMALL-CELL LUNG CANCER (SEVENTH EDITION) |
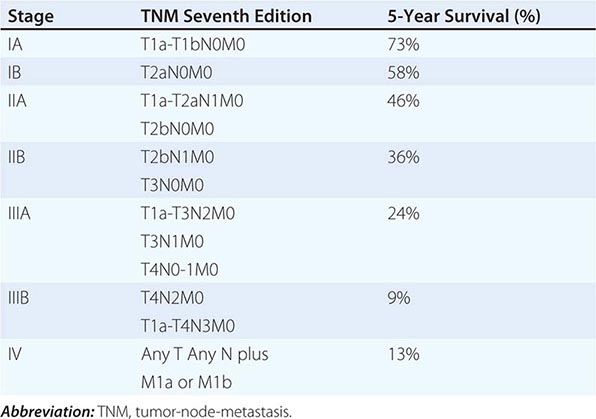
STAGING SYSTEM FOR SMALL-CELL LUNG CANCER
In patients with SCLC, it is now recommended that both the Veterans Administration system and the American Joint Committee on Cancer/International Union Against Cancer seventh edition system (TNM) be used to classify the tumor stage. The Veterans Administration system is a distinct two-stage system dividing patients into those with limited- or extensive-stage disease. Patients with limited-stage disease (LD) have cancer that is confined to the ipsilateral hemithorax and can be encompassed within a tolerable radiation port. Thus, contralateral supraclavicular nodes, recurrent laryngeal nerve involvement, and superior vena caval obstruction can all be part of LD. Patients with extensive-stage disease (ED) have overt metastatic disease by imaging or physical examination. Cardiac tamponade, malignant pleural effusion, and bilateral pulmonary parenchymal involvement generally qualify disease as ED, because the involved organs cannot be encompassed safely or effectively within a single radiation therapy port. Sixty to 70% of patients are diagnosed with ED at presentation. The TNM staging system is preferred in the rare SCLC patient presenting with what appears to be clinical stage I disease (see above).
PHYSIOLOGIC STAGING
Patients with lung cancer often have other comorbid conditions related to smoking including cardiovascular disease and COPD. To improve their preoperative condition, correctable problems (e.g., anemia, electrolyte and fluid disorders, infections, cardiac disease, and arrhythmias) should be addressed, appropriate chest physical therapy should be instituted, and patients should be encouraged to stop smoking. Because it is not always possible to predict whether a lobectomy or pneumonectomy will be required until the time of operation, a conservative approach is to restrict surgical resection to patients who could potentially tolerate a pneumonectomy. Patients with a forced expiratory volume in 1 s (FEV1) of greater than 2 L or greater than 80% of predicted can tolerate a pneumonectomy, and those with an FEV1 greater than 1.5 L have adequate reserve for a lobectomy. In patients with borderline lung function but a resec-table tumor, cardiopulmonary exercise testing could be performed as part of the physiologic evaluation. This test allows an estimate of the maximal oxygen consumption (VO2max). A VO2max <15 mL/(kg⋅min) predicts for a higher risk of postoperative complications. Patients deemed unable to tolerate lobectomy or pneumonectomy from a pulmonary functional standpoint may be candidates for more limited resections, such as wedge or anatomic segmental resection, although such procedures are associated with significantly higher rates of local recurrence and a trend toward decreased overall survival. All patients should be assessed for cardiovascular risk using American College of Cardiology and American Heart Association guidelines. A myocardial infarction within the past 3 months is a contraindication to thoracic surgery because 20% of patients will die of reinfarction. An infarction in the past 6 months is a relative contraindication. Other major contraindications include uncontrolled arrhythmias, an FEV1 of less than 1 L, CO2 retention (resting PCO2 >45 mmHg), DLCO <40%, and severe pulmonary hypertension.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree