Lobular Carcinoma In Situ
Key Facts
Terminology
Lobular carcinoma in situ (LCIS)
Etiology/Pathogenesis
Hallmark molecular feature of in situ and invasive lobular carcinoma is loss of cellular cohesion
Loss of functional E-cadherin protein is most common, followed by defects in catenins
Clinical Issues
LCIS is often found as multiple foci within either the same or both breasts
Risk of subsequent invasive carcinoma is approximately 1% per year for both breasts
Higher incidence of lobular carcinomas, but majority are of no special type
Clinical follow-up should be long-term, given extended risk for late-occurring carcinoma
Microscopic Pathology
Classic LCIS
Solid and monotonous proliferation of small discohesive cells expanding terminal duct lobular units and small ducts
ER and PR positive, HER2 negative
Variants of LCIS
Variant LCIS has morphologic and molecular features not present in classic LCIS
May be ER or PR negative &/or HER2 positive
Consistent terminology for these lesions has not yet been developed
Natural history and optimal treatment is unknown
TERMINOLOGY
Abbreviations
Lobular carcinoma in situ (LCIS)
Synonyms
Lobular intraepithelial neoplasia (LIN), includes atypical lobular hyperplasia (ALH) and LCIS
Definitions
Neoplastic proliferation of epithelial cells lacking cell adhesion, confined to ducts and lobules, and filling and expanding at least 1/2 of 1 lobular unit
ETIOLOGY/PATHOGENESIS
Molecular Pathology
Hallmark molecular feature of all lobular neoplasia (ALH, LCIS, and ILC) is loss of cellular cohesion
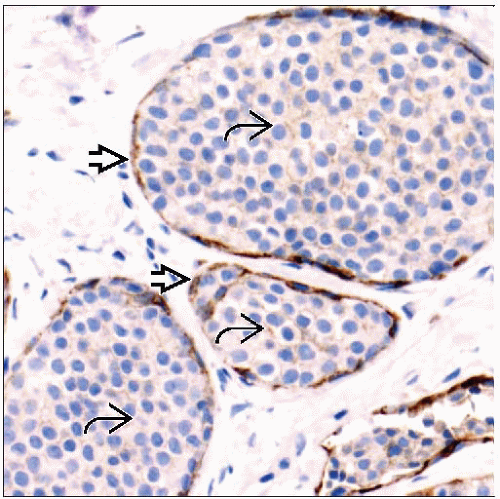
In > 95% of lesions, this is due to loss of expression of E-cadherin (CDH11) located on chromosome 16q
E-cadherin plays major role in intercellular adhesion and cell polarity
Cytoplasmic portion of E-cadherin is attached to actin cytoskeleton by binding β- or γ-catenin
Absence of E-cadherin is due to combinations of mechanisms leading to biallelic inactivation of gene
Allelic deletion (loss of heterozygosity 16q), CDH1 gene mutation, promoter silencing (hypermethylation)
In rare cases, loss of cohesion is due to defects in catenins or possibly other related proteins
Carcinomas have typical morphology, but nonfunctional E-cadherin is present
Other proteins, such as catenins, may have abnormal expression patterns
Distribution of LCIS suggests association with germline mutation
LCIS is not specifically associated with mutations in BRCA1, BRCA2, or CDH11 (E-cadherin)
Families with germline mutations in CDH11 are predominantly at risk for signet ring cell carcinomas of the stomach, with fewer cases of lobular carcinoma
Association with other genes is being investigated
CLINICAL ISSUES
Epidemiology
Incidence
0.5-4% of breast biopsies
Age
Mean age: 44-46 years; 80-90% are premenopausal
Site
LCIS is frequently found as multiple foci within the same or both breasts
Up to 50% of patients have bilateral LCIS
Multicentric foci have been described in up to 80% of patients undergoing mastectomy for LCIS
Presentation
Classic LCIS is incidental finding
Does not form palpable mass or mammographic lesion
Variant forms of LCIS may be associated with calcifications detected by mammography
Natural History
LCIS, ALH, and flat epithelial atypia/columnar cell change share similar molecular and cytogenetic features
Columnar cell change often coexists with LCIS
May be part of morphologic continuum in low-grade neoplasia pathway
Genetic studies have found similar or identical mutations in LCIS and corresponding invasive lobular carcinomas
Similarities between LCIS and invasive carcinoma provide evidence that LCIS can be direct precursor of invasive carcinoma
Natural history for variants of LCIS is unknown
Treatment
Surgical approaches
Due to multicentric nature of LCIS, effective risk reduction requires bilateral mastectomy
However, majority of women with LCIS will never develop invasive carcinoma
Adjuvant therapy
Adjuvant endocrine therapy (chemoprevention) reduces subsequent risk of ER-positive carcinomas
Associated with significant side effects in some women
Surveillance
Women can be followed with close surveillance using imaging modalities
Risk of dying of breast cancer with careful follow-up is very low
Optimal treatment for variants of LCIS is unclear
Some authors feel that variant forms of LCIS are better managed like DCIS
Prognosis
Increased long-term risk of subsequent invasive carcinoma after diagnosis of classic LCIS
Subsequent invasive carcinomas more likely to be lobular than in general population but are most commonly of no special type
Magnitude of risk after variant types of LCIS is unknown but is presumed to be higher
Risk applies to both breasts
Slightly greater in ipsilateral than in contralateral breast
Relative risk is 7-10x or about 25-30% lifetime risk
Corresponds to approximately 1% of patients diagnosed with invasive carcinoma per year of follow-up
Time to cancer from LCIS diagnosis ranges from 15-30 years
Clinical follow-up should be long-term, given extended risk for late-occurring carcinoma
Some studies suggest risk for development of subsequent cancer diminishes with time whereas other studies do not
LCIS on Core Needle Biopsy
Classic LCIS may be seen as incidental finding in core needle biopsies
Excision is indicated for variant types of LCIS if other lesions of risk are present (e.g., ADH) or if targeted lesion may have been missed or is not well explained by pathologic findings
Appropriate management of incidental classic LCIS on core biopsy is debated
In carefully selected cases, frequency of finding more significant lesions after excision is very low
Subsequent cancers in these patients are often not at site of core needle biopsy
IMAGE FINDINGS
Mammographic Findings
Classic LCIS is not associated with mammographic findings
Usually incidental finding in biopsies performed for calcifications
Usually seen in coexisting sclerosing adenosis or columnar cell change adjacent to LCIS
LCIS may grow into areas of preexisting calcifications but are not source of calcifications
Variant forms of LCIS with necrosis &/or high-grade nuclei may be directly associated with calcifications
MR Findings
Some lesions may show a non-mass ductal enhancement pattern or irregular enhancing mass
However, difficult to correlate pathologic findings with MR lesions
MICROSCOPIC PATHOLOGY
Histologic Features
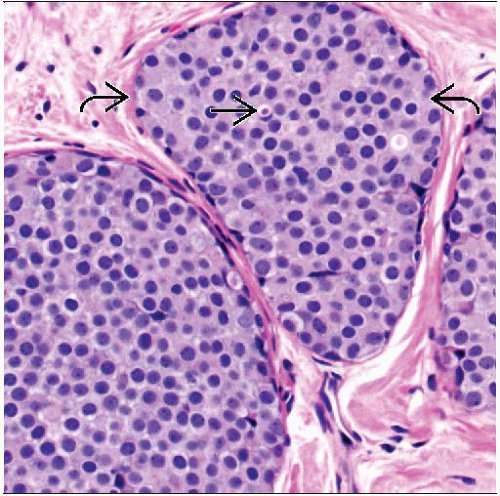
Classic LCIS
Solid and monotonous proliferation of small discohesive cells, expanding terminal duct lobular units (TDLUs) and small ducts
Criterion to distinguish LCIS from ALH is based on extent
At least 50-75% of acini in lobular unit must be filled and distended with no residual lumina
Involved lobules may be compared to uninvolved lobules to estimate degree of distension
2 types of nuclei may be seen
Type A cells: Nuclei are small to slightly enlarged (1-1.5x size of lymphocyte), with uniform round nuclei and inconspicuous nucleoli
Type B cells: Nuclei are larger (2x size of lymphocyte) with more abundant cytoplasm and more prominent nucleoli
Type A and B cells can coexist in same lesion
Nuclei have a central or slightly eccentric position within the cell and show minimal pleomorphism
Intracytoplasmic vacuoles and signet ring cells are variably present
Special stains can be used to demonstrate mucin vacuoles
Cytoplasmic clearing can also be seen
Pagetoid spread in ducts (growth of cells between luminal and myoepithelial layers) may be present
Extensive ductal involvement can give “clover leaf” pattern
Variants of LCIS
Recognition of E-cadherin loss as consistent feature of lobular neoplasia broadened range of lesions included within this group
Often associated with classic LCIS
Occur in older postmenopausal age group compared with classic LCIS
May present as mammographic calcifications or a mass
Many of these lesions would have been classified and treated as DCIS in the past
These lesions are rare, comprising < 5% of all carcinoma in situ
Natural history is unknown
Variant LCIS has morphologic features not seen in classic LCIS
High nuclear grade: > 3x size of a lymphocyte, irregular membrane, prominent nucleoli
Central necrosis, typically with calcifications
Abundant cytoplasm often with apocrine or plasmacytoid appearance
Cohesive (solid) pattern
Variant LCIS has molecular features not seen in classic LCIS
May be ER and PR negative
About 1/3 overexpress HER2
Higher proliferative rate compared with classic LCIS
Share genetic changes with classic LCIS (gains of 1q and losses of 16q) but have a higher number of DNA copy number changes and more complex chromosomal rearrangements
Associated invasive carcinomas are similar in appearance and may belong to “molecular apocrine” group of cancers
Consistent terminology for these lesions has not yet been developed, but suggested terms include
Pleomorphic LCIS
Carcinoma in situ with ductal and lobular features
Lobular intraepithelial neoplasia grade 3 (LIN3)
Apocrine pleomorphic lobular carcinoma in situ
Mammary intraepithelial neoplasia (MIN)
ANCILLARY TESTS
Immunohistochemistry
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree