Chapter 7 Liver, biliary tract and pancreatic disease
The liver
Structure of the liver and biliary system
The liver
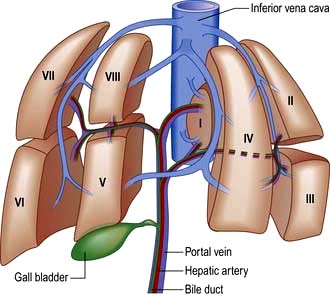
The liver is the body’s largest internal organ (1.2–1.5 kg) and is situated in the right hypochondrium. A functional division into the larger right lobe (containing caudate and quadrate lobes) and the left lobe is made by the middle hepatic vein. The liver is further subdivided into eight segments (Fig. 7.1) by divisions of the right, middle and left hepatic veins. Each segment has its own portal pedicle, permitting individual segment resection at surgery.
 The hepatic artery, a branch of the coeliac axis, supplies 25% of the hepatic blood flow. The hepatic artery autoregulates flow ensuring a constant total blood flow.
The hepatic artery, a branch of the coeliac axis, supplies 25% of the hepatic blood flow. The hepatic artery autoregulates flow ensuring a constant total blood flow.
 The portal vein drains most of the gastrointestinal tract and the spleen. It supplies 75% of hepatic blood flow. The normal portal pressure is 5–8 mmHg; flow increases after meals.
The portal vein drains most of the gastrointestinal tract and the spleen. It supplies 75% of hepatic blood flow. The normal portal pressure is 5–8 mmHg; flow increases after meals.
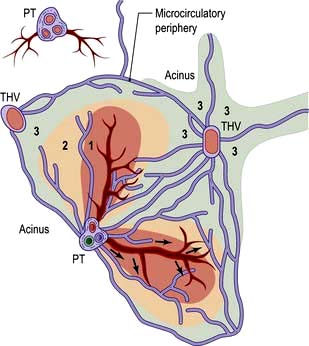
The acinus is the functional hepatic unit. This consists of parenchyma supplied by the smallest portal tracts containing portal vein radicles, hepatic arterioles and bile ductules (Fig. 7.2). The hepatocytes near this triad (zone 1) are well supplied with oxygenated blood and are more resistant to damage than the cells nearer the terminal hepatic (central) veins (zone 3).
The sinusoids lack a basement membrane and are loosely surrounded by specialist fenestrated endothelial cells and Kupffer cells (phagocytic cells). Sinusoids are separated by plates of liver cells (hepatocytes). The subendothelial space between the sinusoids and hepatocytes is the space of Disse, which contains a matrix of basement membrane constituents and stellate cells (see Fig. 7.23).
Stellate cells store retinoids in their resting state and contain the intermediate filament, desmin. When activated (to myofibroblasts) they are contractile and probably regulate sinusoidal blood flow. Endothelin and nitric oxide play a major role in modulating stellate cell contractility. Activated stellate cells produce signal proteins for synthesis or inhibition of degradation of extracellular matrix components, including collagen, as well as cytokines and chemotactic signals (see p. 328).
Functions of the liver
Protein metabolism (see also p. 197)
Synthesis and storage
The liver also synthesizes all factors involved in coagulation (apart from one-third of factor VIII) – that is, fibrinogen, prothrombin, factors V, VII, IX, X and XIII, proteins C and S and antithrombin (see Ch. 8) as well as components of the complement system. The liver stores large amounts of vitamins, particularly A, D and B12, and lesser amounts of others (vitamin K and folate), and also minerals – iron in ferritin and haemosiderin and copper.
Carbohydrate metabolism
Glucose homeostasis and the maintenance of the blood sugar is a major function of the liver. It stores approximately 80 g of glycogen. In the immediate fasting state, blood glucose is maintained either by glucose release from breaking down glycogen (glycogenolysis) or by synthesizing new glucose (gluconeogenesis). Sources for gluconeogenesis are lactate, pyruvate, amino acids from muscles (mainly alanine and glutamine) and glycerol from lipolysis of fat stores. In prolonged starvation, ketone bodies and fatty acids are used as alternative sources of fuel as the body tissues adapt to a lower glucose requirement (see Ch. 5).
Lipid metabolism
Fats are insoluble in water and are transported in plasma as protein-lipid complexes (lipoproteins). These are discussed in detail on page 1005.
The liver has a major role in metabolizing of lipoproteins. It synthesizes very-low-density lipoproteins (VLDLs) and high-density lipoproteins (HDLs). HDLs are the substrate for lecithin-cholesterol acyltransferase (LCAT), which catalyses the conversion of free cholesterol to cholesterol ester (see below). Hepatic lipase removes triglyceride from intermediate-density lipoproteins (IDLs) to produce low-density lipoproteins (LDLs) which are degraded by the liver after uptake by specific cell-surface receptors (see Fig. 20.19).
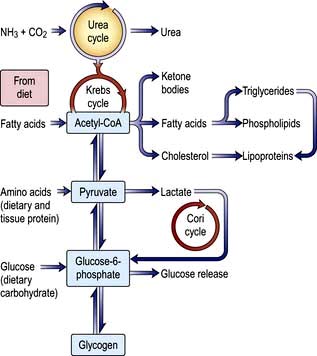
Cholesterol may be of dietary origin but most is synthesized from acetyl-CoA mainly in the liver, intestine, adrenal cortex and skin. It occurs either as free cholesterol or is esterified with fatty acids; this reaction is catalysed by LCAT. This enzyme is reduced in severe liver disease, increasing the ratio of free cholesterol to ester, which alters membrane structures. One result of this is the red cell abnormalities (e.g. target cells) seen in chronic liver disease. Phospholipids (e.g. lecithin) are synthesized in the liver. The complex interrelationships between protein, carbohydrate and fat metabolism are shown in Figure 7.3.
Formation of bile
Bile secretion and bile acid metabolism
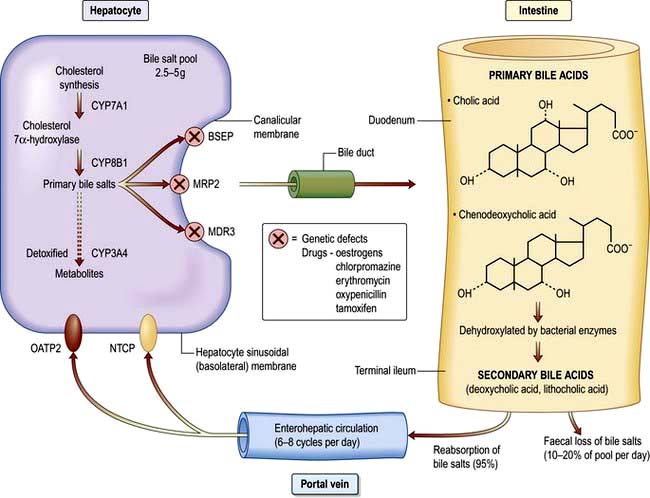
Bile formation requires uptake of bile acids and other organic and inorganic ions across the basolateral (sinusoidal) membranes by multiple transport proteins (sodium taurocholate co-transporting polypeptide (NTCP) and sodium independent organic anion transporting polypeptide 2 (OATP2), Fig. 7.4). This process is driven by Na+/K+-ATPase in the basolateral membranes. Intracellular transport across hepatocytes is partly through microtubules and partly by cytosol transport proteins.
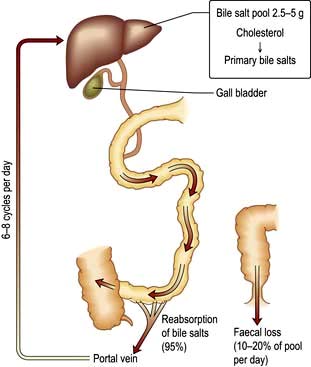
The bile acids are excreted into bile and then pass via the common bile duct into the duodenum. The two primary bile acids – cholic acid and chenodeoxycholic acid (Fig. 7.4) – are conjugated with glycine or taurine, which increases their solubility. Intestinal bacteria convert these acids into secondary bile acids, deoxycholic and lithocholic acid. Figure 7.5 shows the enterohepatic circulation of bile acids.
Bilirubin metabolism
Normally, 250–300 mg (425–510 mmol) of bilirubin are produced daily. The iron and globin are removed from haem and are reused. Biliverdin is formed from haem and then reduced to form bilirubin. The bilirubin produced is unconjugated and water-insoluble, due to internal hydrogen bonding, and is transported to the liver attached to albumin. Bilirubin dissociates from albumin and is taken up by hepatic cell membranes and transported to the endoplasmic reticulum by cytoplasmic proteins, where it is conjugated with glucuronic acid and excreted into bile. The microsomal enzyme, uridine diphosphoglucuronosyl transferase, catalyses the formation of bilirubin monoglucuronide and then diglucuronide. This conjugated bilirubin is water-soluble and is actively secreted into biliary canaliculi and excreted into the intestine within bile (Fig. 8.5). It is not absorbed from the small intestine because of its large molecular size. In the terminal ileum, bacterial enzymes hydrolyse the molecule, releasing free bilirubin which is then reduced to urobilinogen, some of which is excreted in the stools as stercobilinogen. The remainder is absorbed by the terminal ileum, passes to the liver via the enterohepatic circulation, and is re-excreted into bile. Urobilinogen bound to albumin enters the circulation and is excreted in urine via the kidneys. When hepatic excretion of conjugated bilirubin is impaired, a small amount is strongly bound to serum albumin and is not excreted by the kidneys; it accounts for the persistent hyperbilirubinaemia for a time after cholestasis has resolved.
Hormone and drug inactivation
The liver catabolizes hormones such as insulin, glucagon, oestrogens, growth hormone, glucocorticoids and parathyroid hormone. It is also the prime target organ for many hormones (e.g. insulin). It is the major site for the metabolism of drugs (see p. 348) and alcohol (see p. 225). Fat-soluble drugs are converted to water-soluble substances that facilitate their excretion in the bile or urine. Cholecalciferol is converted to 25-hydroxycholecalciferol.
Investigations
Investigative tests can be divided into:
 Urine tests – for bilirubin and urobilinogen
Urine tests – for bilirubin and urobilinogen
 Imaging techniques – to define gross anatomy
Imaging techniques – to define gross anatomy
Blood tests
Useful blood tests for certain liver diseases are shown in Table 7.1.
Table 7.1 Useful blood and urine tests for certain liver diseases
| Test | Disease |
|---|---|
Anti-mitochondrial antibody | Primary biliary cirrhosis |
Anti-nuclear, smooth muscle (actin), liver/kidney microsomal antibody | Autoimmune hepatitis |
Raised serum immunoglobulins: |
|
IgG | Autoimmune hepatitis |
IgM | Primary biliary cirrhosis |
Viral markers | Hepatitis A, B, C, D, E and others |
α-Fetoprotein | Hepatocellular carcinoma |
Serum iron, transferrin saturation, serum ferritin | Hereditary haemochromatosis |
Serum and urinary copper, serum caeruloplasmin | Wilson’s disease |
α1-Antitrypsin | Cirrhosis (± emphysema) |
Anti-nuclear cytoplasmic antibodies | Primary sclerosing cholangitis |
Markers of liver fibrosis | Non-alcoholic fatty liver disease |
| Hepatitis C |
Genetic analyses | e.g. HFE gene (hereditary haemochromatosis) |
Liver biochemistry
 Aspartate aminotransferase (AST) is primarily a mitochondrial enzyme (80%; 20% in cytoplasm) and is also present in heart, muscle, kidney and brain. High levels are seen in hepatic necrosis, myocardial infarction, muscle injury and congestive cardiac failure.
Aspartate aminotransferase (AST) is primarily a mitochondrial enzyme (80%; 20% in cytoplasm) and is also present in heart, muscle, kidney and brain. High levels are seen in hepatic necrosis, myocardial infarction, muscle injury and congestive cardiac failure.
 Alanine aminotransferase (ALT) is a cytosol enzyme, more specific to the liver so that a rise only occurs with liver disease.
Alanine aminotransferase (ALT) is a cytosol enzyme, more specific to the liver so that a rise only occurs with liver disease.
This is a microsomal enzyme present in liver, but also in many tissues. Its activity can be induced by drugs such as phenytoin and by alcohol. If the ALP is normal, a raised serum γ-GT can be a useful guide to alcohol intake (see p. 1182). However, mild elevations of γ-GT are common, even with a small alcohol consumption and is also raised with fatty liver disease. It does not necessarily indicate liver disease if the other liver biochemical tests are normal. In cholestasis the γ-GT rises in parallel with the ALP as it has a similar pathway of excretion. This is also true of 5-nucleotidase, another microsomal enzyme that can be measured in blood.
Additional blood investigations
Haematological
 Bleeding produces a hypochromic, microcytic picture.
Bleeding produces a hypochromic, microcytic picture.
 Alcohol causes macrocytosis, sometimes with leucopenia and thrombocytopenia.
Alcohol causes macrocytosis, sometimes with leucopenia and thrombocytopenia.
 Hypersplenism results in pancytopenia.
Hypersplenism results in pancytopenia.
 Cholestasis can often produce abnormal-shaped cells, and also deficiency of vitamin K.
Cholestasis can often produce abnormal-shaped cells, and also deficiency of vitamin K.
 Haemolysis may accompany acute liver failure and jaundice.
Haemolysis may accompany acute liver failure and jaundice.
 Aplastic anaemia occurs in up to 2% of patients with acute viral hepatitis.
Aplastic anaemia occurs in up to 2% of patients with acute viral hepatitis.
 A raised serum ferritin with transferrin saturation (>60%) is seen in hereditary haemochromatosis.
A raised serum ferritin with transferrin saturation (>60%) is seen in hereditary haemochromatosis.
Biochemical
 a1-Antitrypsin. A deficiency of this enzyme can produce cirrhosis.
a1-Antitrypsin. A deficiency of this enzyme can produce cirrhosis.
 α-Fetoprotein. This is normally produced by the fetal liver. Its reappearance in increasing and high concentrations in adults indicates hepatocellular carcinoma. Increased concentrations in pregnancy in blood and amniotic fluid suggest fetal neural tube defects. Blood levels are also slightly raised with regenerative liver tissue in patients with hepatitis, chronic liver disease and also in teratomas.
α-Fetoprotein. This is normally produced by the fetal liver. Its reappearance in increasing and high concentrations in adults indicates hepatocellular carcinoma. Increased concentrations in pregnancy in blood and amniotic fluid suggest fetal neural tube defects. Blood levels are also slightly raised with regenerative liver tissue in patients with hepatitis, chronic liver disease and also in teratomas.
 Raised urinary copper, and low serum cooper and caeruloplasmin in Wilson’s disease (see p. 341).
Raised urinary copper, and low serum cooper and caeruloplasmin in Wilson’s disease (see p. 341).
Immunological tests
 Anti-mitochondrial antibody (AMA) in serum is found in over 95% of patients with primary biliary cirrhosis (PBC) (p. 338). Several different AMA subtypes are described, depending on their antigen specificity, and are also found in autoimmune hepatitis and other autoimmune diseases. AMA is demonstrated by an immunofluorescent technique and is neither organ- nor species-specific. M2 subtype is specific for PBC.
Anti-mitochondrial antibody (AMA) in serum is found in over 95% of patients with primary biliary cirrhosis (PBC) (p. 338). Several different AMA subtypes are described, depending on their antigen specificity, and are also found in autoimmune hepatitis and other autoimmune diseases. AMA is demonstrated by an immunofluorescent technique and is neither organ- nor species-specific. M2 subtype is specific for PBC.
 Nucleic, smooth muscle (actin), liver/kidney microsomal antibodies can be found in serum, often in high titre, in patients with autoimmune hepatitis. These serum antibodies can also be found in other autoimmune conditions and other liver diseases.
Nucleic, smooth muscle (actin), liver/kidney microsomal antibodies can be found in serum, often in high titre, in patients with autoimmune hepatitis. These serum antibodies can also be found in other autoimmune conditions and other liver diseases.
 Anti-nuclear cytoplasmic antibodies (ANCA) can be found in serum of patients with primary sclerosing cholangitis.
Anti-nuclear cytoplasmic antibodies (ANCA) can be found in serum of patients with primary sclerosing cholangitis.
Genetic analysis
These tests are performed routinely for haemochromatosis (HFE gene) and for α1-antitrypsin deficiency. Markers are also available for the most frequent abnormal genes in Wilson’s disease (see p. 341).
Imaging techniques
Ultrasound examination
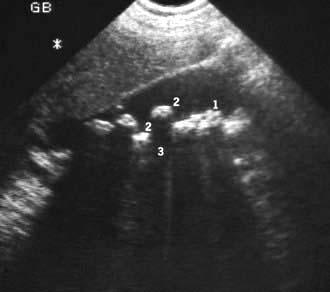
 the detection of gallstones (Fig. 7.6)
the detection of gallstones (Fig. 7.6)
 focal liver disease – lesions >1 cm
focal liver disease – lesions >1 cm
 general parenchymal liver disease
general parenchymal liver disease
 assessing portal and hepatic vein patency
assessing portal and hepatic vein patency
Other abdominal masses can be delineated and biopsies obtained under ultrasonic guidance.
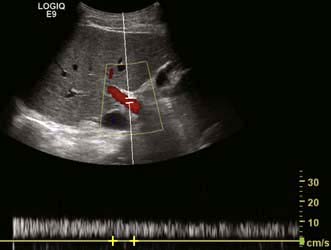
Colour Doppler ultrasound will demonstrate vascularity within a lesion and the direction of portal and hepatic vein blood flow (Fig. 7.7).
Computed tomography (CT) examination
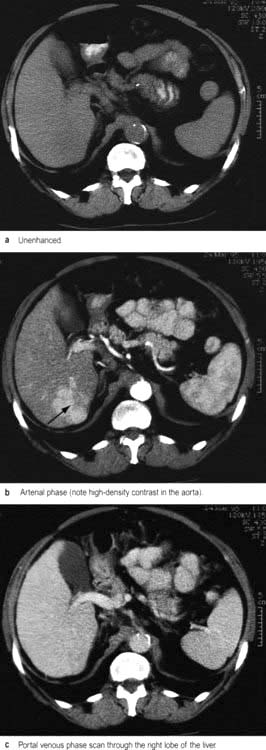
CT during or immediately after i.v. contrast shows both arterial and portal venous phases of enhancement, enabling more precise characterization of a lesion and its vascular supply (Fig. 7.8). Retrospective analysis of data allows multiple overlapping slices to be obtained with no increase in the radiation dose, providing excellent visualization of the size, shape and density of the liver, pancreas, spleen, lymph nodes and lesions in the porta hepatis. Multi-planar and three-dimensional reconstruction in the arterial phase can create a CT angiogram, often making formal invasive angiography unnecessary. CT also provides guidance for biopsy. It has advantages over US in detecting calcification and is useful in obese subjects, although US is usually the imaging modality used first to investigate liver disease.
Endoscopic retrograde cholangiopancreatography (ERCP)
 Common bile duct stones can be removed after performing a diathermy cut of the sphincter to facilitate their withdrawal. Sphincterotomy has a morbidity rate of 3–5%: acute pancreatitis is the commonest, severe haemorrhage is rare. There is an overall mortality of 0.4%.
Common bile duct stones can be removed after performing a diathermy cut of the sphincter to facilitate their withdrawal. Sphincterotomy has a morbidity rate of 3–5%: acute pancreatitis is the commonest, severe haemorrhage is rare. There is an overall mortality of 0.4%.
 The biliary system can be drained by passing a tube (stent) through an obstruction, or placement of a nasobiliary drain.
The biliary system can be drained by passing a tube (stent) through an obstruction, or placement of a nasobiliary drain.
 Brachytherapy can be administered after placement at ERCP for therapy of cholangiocarcinoma.
Brachytherapy can be administered after placement at ERCP for therapy of cholangiocarcinoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree