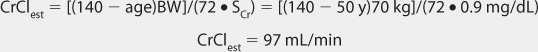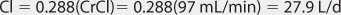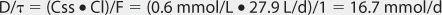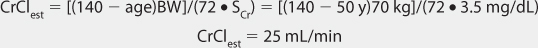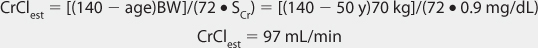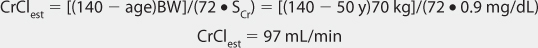FIGURE 21-1 Lithium ion serum concentration-time curve after a single 900-mg oral dose of lithium carbonate (24.4 mmol or mEq of lithium ion) rapid-release capsules. Maximum serum concentrations occur 2-3 hours after the dose is given. After the peak concentration is achieved, the distribution phase lasts for 6-10 hours, followed by the elimination phase. In patients with good renal function (creatinine clearance >80 mL/min), the average elimination half-life for lithium is 24 hours. Because of the long distribution phase, lithium serum concentrations used for dosage adjustment purposes should be obtained no sooner than 12 hours after dosage administration.
At lithium serum concentrations within the upper end of the therapeutic range (1.2-1.5 mmol/L), the following adverse effects can be noted in patients: decreased memory and concentration, drowsiness, fine hand tremor, weakness, lack of coordination, nausea, diarrhea, vomiting, or fatigue.1,2 At concentrations just above the therapeutic range (1.5-3 mmol/L), confusion, giddiness, agitation, slurred speech, lethargy, blackouts, ataxia, dysarthria, nystagmus, blurred vision, tinnitus, vertigo, hyperreflexia, hypertonia, dysarthria, coarse hand tremors, and muscle fasciculations may occur in patients. If concentrations exceed 3 mmol/L, severe toxicity occurs with choreoathetosis, seizures, irreversible brain damage, arrhythmias, hypotension, respiratory and cardiovascular complications, stupor, coma, and death. At toxic lithium concentrations, lithium can cause a nonspecific decrease in glomerular filtration which, in turn, decreases lithium clearance. The decrease in lithium clearance will cause a further increase in the lithium serum concentration. This phenomenon can cause a viscous circle of decreased clearance leading to increased lithium serum concentration, which leads to additional decreases in lithium clearance and so on. Because of this and the severe toxic side effects, lithium concentrations above 3.5-4 mmol/L may require hemodialysis to remove the drug as quickly as possible.1,2,4
CLINICAL MONITORING PARAMETERS
The signs and symptoms of bipolar disease include both those of depression (depressed affect, sad mood, decreased interest and pleasure in normal activities, decreased appetite and weight loss, insomnia or hypersomnia, psychomotor retardation or agitation, decreased energy or fatigue, feelings of worthlessness or guilt, impaired decision making and concentration, suicidal ideation or attempts) and mania (abnormal and persistently elevated mood, grandiosity, decreased need for sleep, pressure of speech, flight of ideas, distractible with poor attention span, increased activity or agitation, excessive involvement in high-risk activities).1,2,4 Generally, onset of action for lithium is 1-2 weeks, and a 4- to 6-week treatment period is required to assess complete therapeutic response to the drug.1,2,4
Before initiating lithium therapy, patients should undergo a complete physical examination, and a general serum chemistry panel (including serum electrolytes and serum creatinine), complete blood cell count with differential, thyroid function tests, urinalysis (including osmolality and specific gravity), and urine toxicology screen for substances of abuse should also be obtained. For patients with renal dysfunction (measured 24-hour creatinine clearance) or baseline cardiac disease (electrocardiogram) additional testing is recommended. Clinicians should consider ordering a pregnancy test for females of childbearing age. Follow-up testing in the following areas should be conducted every 6-12 months: serum electrolytes, serum creatinine (measured 24-hour creatinine clearance in patients with renal dysfunction), thyroid function tests, complete blood cell count with differential. If urine output exceeds 3 L/d, a urinalysis with osmolality and specific gravity should also be measured.
Lithium serum concentrations should be measured in every patient receiving the drug. As previously discussed, dosage schedules should be arranged so that serum samples for lithium measurement are obtained 12 ± 0.5 hours after a dose.5 Usually this requires administration of the drug every 12 hours for twice daily dosing. For three times a day dosing, it is necessary to give the drug so that there is a 12-hour time period overnight. Examples of two common dosage schemes are 0900 H, 1500 H, and 2100 H or 0800 H, 1400 H, and 2000 H. Obviously, the choice should be individualized based upon the patient’s lifestyle. Upon initiation of therapy, serum concentrations can be measured every 2-3 days for safety reasons in patients that are predisposed to lithium toxicity even though steady-state has not yet been achieved. Once the desired steady-state lithium concentration has been achieved, lithium concentrations should be rechecked every 1-2 weeks for approximately 2 months or until concentrations have stabilized. Because patients with acute mania can have increased lithium clearance, lithium concentrations should be remeasured in these patients once the manic episode is over and clearance returns to normal. Otherwise, lithium concentrations may accumulate to toxic levels due to the decrease in lithium clearance. During lithium maintenance therapy, steady-state lithium serum concentrations should be repeated every 3-6 months. This time period should be altered to every 6-12 months for patients whose mood is stable or every 1-2 months for patients with frequent mood alterations. If lithium dosage alterations are needed, or therapy with another drug known to interact with lithium is added, lithium serum concentrations should be measured within 1-2 weeks after the change.
After patients have been stabilized on a multiple dose per day regimen, it is possible to consider once daily administration of lithium for those receiving a total dose of 1800 mg/d or less.4 However, the change in dosage interval will alter the 12 hour lithium concentration, and further dosage titration may be needed to re-establish desired levels.4
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Lithium is eliminated almost completely (>95%) unchanged in the urine.9 The ion is filtered freely at the glomerulus, and subsequently 60%-80% of the amount filtered is reabsorbed by the proximal tubule of the nephron. Lithium eliminated in the saliva, sweat, and feces accounts for less than 5% of the administered dose.10 On average, lithium clearance is approximately 20% of the patient’s creatinine clearance.10–12 Lithium is administered orally as carbonate or citrate salts. Lithium carbonate capsules (150, 300, 600 mg) and tablets (rapid release: 300 mg; sustained release: 300, 450 mg) are available. There are 8.12 mmol (or 8.12 mEq) of lithium in 300 mg of lithium carbonate. Lithium citrate syrup (8 mmol or mEq/5 mL) is another oral dosage form. Oral bioavailability is good for all lithium salts and dosage forms and equals 100%.13,14 The peak lithium concentration occurs 15-30 minutes after a dose of lithium citrate syrup, 1-3 hours after a dose of rapid-release lithium carbonate tablets or capsules, and 4-8 hours after a dose of sustained-release lithium carbonate tablets. Lithium ion is not plasma protein bound. The typical dose of lithium carbonate is 900-2400 mg/d in adult patients with normal renal function.
EFFECTS OF DISEASE STATES AND CONDITIONS ON LITHIUM PHARMACOKINETICS
Adults with normal renal function (creatinine clearance >80 mL/min) have an average elimination half-life of 24 hours, volume of distribution equal to 0.9 L/kg, and clearance of 20 mL/min for lithium.5–9 During an acute manic phase, lithium clearance can increase by as much as 50% which produces a half-life that is about ½ the normal value.15 In children 9-12 years of age, average elimination half-life equals 18 hours, volume of distribution is 0.9 L/kg, and clearance equals 40 mL/min for the ion.16 Because glomerular filtration and creatinine clearance decrease with age, lithium clearance can be decreased in elderly patients, producing half-lives up to 36 hours.11,12 Because of the circadian rhythm of glomerular filtration, lithium clearance is about 30% higher during daytime hours.17
Because lithium is eliminated almost exclusively by the kidney, renal dysfunction is the most important disease state that effects lithium pharmacokinetics. Lithium clearance rate decreases in proportion to creatinine clearance. In adults, the lithium clearance-creatinine clearance ratio is 20%, but during a manic phase increases to about 30%.11,12,15 This relationship between renal function and lithium clearance will form the basis for initial dosage computation later in the chapter. Because of the decrease in clearance, the average lithium half-life is 40-50 hours in renal failure patients.
The renal clearance of lithium for a patient is influenced by the state of sodium balance and fluid hydration in that individual. Lithium is reabsorbed in the proximal tubule of the nephron via the same mechanisms used to maintain sodium balance.5 Thus, when a patient is in negative sodium balance, the kidney increases sodium reabsorption as a compensatory maneuver and lithium reabsorption increases as a result. The kidney also increases sodium reabsorption when a patient becomes dehydrated, and, again, lithium reabsorption increases. In both cases, increased lithium reabsorption leads to decreased lithium clearance. Some common things that cause sodium depletion and/or dehydration include sodium-restricted diets for the treatment of other conditions; vomiting, diarrhea, or fever that might be due to viral or other illnesses; heavy or intense exercise; excessive sweating; use of saunas or hot tubs; and hot weather. Overuse of coffee, tea, soft drinks, or other caffeine-containing liquids and ethanol should be avoided by patients taking lithium. Patients should be advised to maintain adequate fluid intake at all times (2.5-3 L/d) and to increase fluid intake as needed.2
During periods of acute mania, lithium clearance can be increased by as much as 50%.15 Lithium is generally not used in the first trimester due to possible teratogenic effects on the fetus.1,2,4 Due to increased glomerular filtration, lithium clearance may be increased in pregnant women, especially during the third trimester. Lithium crosses the placenta, and human milk concentrations are 30%-100% that of concurrent serum concentrations.18
Lithium is removed from the body by hemodialysis, peritoneal dialysis, and arteriovenous hemodiafiltration with clearance values of 30-50 mL/min, 13-15 mL/min, and 21 mL/min, respectively.10,19,20 The sieving coefficient for lithium during hemofiltration is 0.90.21,22 Replacement doses of lithium during dialysis or hemofiltration should be determined using serum concentration monitoring.
DRUG INTERACTIONS
Many diuretics have drug interactions with lithium.23 Thiazide diuretics cause sodium and water depletion which leads to increased sodium reabsorption in the proximal tubule of the kidney as a compensatory mechanism. Since lithium is reabsorbed by the same mechanisms as sodium, lithium reabsorption increases and lithium clearance decreases by 40%-50% during treatment with thiazide diuretics. Other diuretics that work at the site of the distal tubule of the kidney may cause a similar interaction with lithium (chlorthalidone, metolazone). Although there are case reports of loop diuretics causing a similar interaction, there are also reports of no drug interaction between lithium and these agents. Because of this, many clinicians favor the use of a loop diuretic, with careful monitoring of adverse effects and lithium serum concentrations, in patients taking lithium. Amiloride has also been reported to have minimal effects on lithium clearance.
Nonsteroidal anti-inflammatory agents (NSAIDs) also decrease lithium clearance and increase lithium concentrations. The probable mechanism is an NSAID-induced decrease in renal blood flow via inhibition of prostaglandins. Of these agents, sulindac and aspirin appear to have little or no drug interaction with lithium.
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been reported to inhibit the elimination of lithium by an undefined mechanism. Of the two classes of drugs, more documentation exists for the ACEIs where lithium serum concentrations have increased by as much as 200%-300% from pretreatment levels.
Some serotonin-specific reuptake inhibitors (SSRIs) have been reported to cause a serotonergic hyperarousal syndrome when taken in conjunction with lithium. Case report of this problem are currently available for fluoxetine, sertraline, and fluvoxamine. In addition to elevated lithium concentrations, patients have developed stiffness of arms and legs, course tremors, dizziness, ataxia, dysarthric speech, and seizures when taking these SSRI agents with lithium. Although there are also literature reports of these combinations used safely, caution should be exercised when concurrent treatment with SSRIs and lithium is indicated.
Theophylline increases the lithium clearance-creatinine clearance ratio by as much as 58% resulting in an average decrease of 21% in steady-state lithium concentrations. A rare, but severe, drug interaction between lithium and antipsychotic drugs has been reported where patients are more susceptible to the development of extrapyramidal symptoms or irreversible brain damage. Again, although there are reports of using antipsychotic agents and lithium together successfully, patients requiring this combination therapy should be closely monitored for adverse drug reactions.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate lithium therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. However, it is computationally intensive. Literature-based recommended dosing is a very commonly used method to prescribe initial doses of lithium. Doses are based on those that commonly produce steady-state concentrations in the lower end of the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient. Test dose methods use concentrations measured after one or more lithium test doses to rapidly individualize lithium therapy.
Pharmacokinetic Dosing Method
The goal of initial dosing of lithium is to compute the best dose possible for the patient given their set of disease states and conditions that influence lithium pharmacokinetics and the type and severity of their bipolar disease. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Clearance Estimate
Lithium ion is almost totally eliminated unchanged in the urine, and there is a consistent relationship between lithium clearance and creatinine clearance with a ratio of 20% between the two (lithium clearance-creatinine clearance).10–12 This relationship allows the estimation of lithium clearance for a patient which can be used to compute an initial dose of the drug. Mathematically, the equation for the straight line shown in Figure 21-2 is: Cl = 0.2(CrCl), where Cl is lithium clearance in mL/min and CrCl is creatinine clearance in mL/min. For dosing purposes, it is more useful to have lithium clearance expressed in L/d. The equation converted to these units is: Cl = 0.288(CrCl), where Cl is lithium clearance in L/d and CrCl is creatinine clearance in mL/min. For patients with acute mania, lithium clearance is increased by about 50%, and the corresponding equation for these individuals is: Cl = 0.432(CrCl), where Cl is lithium clearance in L/d and CrCl is creatinine clearance in mL/min.15
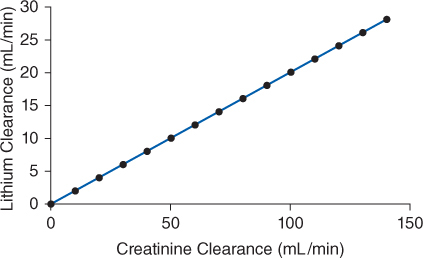
FIGURE 21-2 The ratio between lithium clearance and creatinine clearance is 0.2 for patients requiring maintenance therapy with lithium. This relationship is used to estimate lithium clearance for patients requiring initial dosing with the drug.
Selection of Appropriate Pharmacokinetic Model and Equation
When given orally, lithium follows a two-compartment model (see Figure 21-1).5–9 After the peak concentration is achieved, serum concentrations drop rapidly because of distribution of drug from blood to tissues (α or distribution phase). By 6-10 hours after administration of the drug, lithium concentrations decline more slowly, and the elimination rate constant for this segment of the concentration-time curve is the one that varies with renal function (β or elimination phase). While this model is the most correct from a strict pharmacokinetic viewpoint, it cannot easily be used clinically because of its mathematical complexity. During the elimination phase of the concentration-time curve, lithium serum concentrations drop very slowly due to the long elimination half-life (24 hours with normal renal function, up to 50 hours with end-stage renal disease). Because of this, a very simple pharmacokinetic equation that computes the average lithium steady-state serum concentration (Css in mmol/L = mEq/L) is widely used and allows maintenance dosage calculation: Css = [F(D/τ)]/Cl or D/τ = (Css • Cl)/F, where F is the bioavailability fraction for the oral dosage form (F = 1 for oral lithium), D is the lithium dose in mmoles, τ is the dosage interval in days, and Cl is lithium clearance in L/d. Because this equation computes lithium ion requirement and lithium carbonate doses are prescribed in mg, the ratio of lithium ion content to lithium carbonate salt (8.12 mmol Li+/300 mg lithium carbonate) is used to convert the result from this equation into a lithium carbonate dose. Total daily amounts of lithium are usually given as near-equally divided doses twice or three times a day, and single doses above 1200 mg/d of lithium carbonate are usually not given in order to avoid gastrointestinal upset.
Steady-State Concentration Selection
Lithium serum concentrations are selected based on the presence or absence of acute mania and titrated to response.3 For individuals with acute mania, a minimum lithium concentration of 0.8 mmol/L is usually recommended. The usual desired range for these individuals is 0.8-1 mmol/L. If patients with acute mania do not respond to these levels, it is necessary to occasionally use lithium concentrations of 1-1.2 mmol/L and in some instances concentrations as high as 1.2-1.5 mmol/L are needed. For long-term maintenance use, the usual desired range is 0.6-0.8 mmol/L. If patients do not respond to these levels during maintenance treatment, occasional use of lithium concentrations equal to 0.9-1 mmol/L is required and in some cases concentrations as high as 1-1.2 mmol/L are necessary to gain an adequate outcome.
Literature-Based Recommended Dosing
Because of the large amount of variability in lithium pharmacokinetics, even when concurrent disease states and conditions are identified, many clinicians believe that the use of standard lithium doses for various situations are warranted. The original computation of these doses was based on the Pharmacokinetic Dosing method described in the previous section, and subsequently modified based on clinical experience. For the treatment of acute mania, initial doses are usually 900-1200 mg/d of lithium carbonate.1,2,4 If the drug is being used for bipolar disease prophylaxis, an initial dose of 600 mg/d lithium carbonate is recommended.1,2,4 In both cases, the total daily dose is given in 2-3 divided daily doses. To avoid adverse side effects, lithium doses are slowly increased by 300-600 mg/d every 2-3 days according to clinical response and lithium serum concentrations. Renal dysfunction is the major condition that alters lithium pharmacokinetics and dosage.24–27 If creatinine clearance is 10-50 mL/min, the prescribed initial dose is 50%-75% of that recommended for patients with normal renal function. For creatinine clearance values below 10 mL/min, the prescribed dose should be 25%-50% of the usual dose in patients with good renal function. Recommended doses for children and adolescents with normal renal function are 15-60 mg/kg/d and 600-1800 mg/d, respectively, with doses administered 3-4 times daily.28
Zetin and associates have developed a multiple regression equation that computes lithium carbonate doses for patients based on hospitalization status, age, gender, and weight of the patient as well as the presence or absence of concurrent tricyclic use by the patient.29,30 However, since renal function was not assessed as an independent parameter in their study population, this dosage method is not presented.
To illustrate the similarities and differences between this method of initial dosage calculation and the Pharmacokinetic Dosing method, the same examples used in the previous section will be used.
Test Dose Methods to Assess Initial Lithium Dosage Requirements
Several methods to assess initial lithium dosage requirement using one or most lithium test doses and one or more lithium serum concentrations are available for clinical use.
Cooper Nomogram
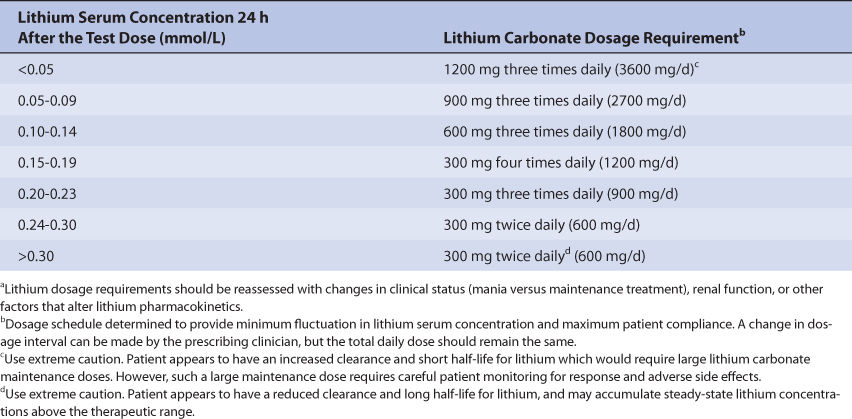
The Cooper nomogram of lithium maintenance dosage assessment requires the administration of a single test dose of 600 mg lithium carbonate and a single lithium serum concentration measured 24 hours later.31,32 The 24-hour lithium serum concentration is compared to a table that converts the observed concentration into the lithium carbonate dose required to produced a steady-state lithium concentration between 0.6 and 1.2 mmol/L (Table 21-1). The theoretical basis for this dosage approach lies in the relationship between the serum concentration of a drug obtained about 1 half-life after dosage and the elimination rate constant for the drug in a patient. This nomogram can also be expressed as an equation for the total daily lithium dosage requirement (D in mmol/d): D = e(4.80 – 7.5Ctest), where Ctest is the 24-hour postdose lithium concentration for a 600-mg lithium carbonate dose.10 Perry and associates have suggested a similar nomogram that employs a larger test dose of 1200 mg lithium carbonate.4,33,34 An important requirement for these methods is an accurate lithium assay that can reproducibly measure the lithium concentrations that occur after a single dose of the drug. Additionally, at the time the lithium carbonate test dose is given, the lithium serum concentration in the patient must equal zero.
TABLE 21-1 Cooper Nomogram for Lithium Dosing31,32
(Lithium Carbonate Dosage Required to Produce Steady-State Lithium Serum Concentrations Between 0.6 and 1.2 mmol/L)a
Perry Method
This technique conducts a small pharmacokinetic experiment in a patient after the administration of a lithium carbonate test dose.35 First, a test dose (600-1500 mg) of lithium carbonate is given to the patient. Then, lithium serum concentrations are measured 12 and 36 hours after the test dose was given. The two lithium concentrations are used to compute the elimination rate constant for the individual: ke = (ln C12h – ln C36h)/Δt, where ke is the elimination rate constant in h–1 for lithium, C12h and C36h are the lithium concentrations in mmol/L (or mEq/L) at 12 and 36 hours, respectively, after the test dose was given, and Δt is the difference between times (24 hours) that the two serum concentrations were obtained. With knowledge of the elimination rate constant (ke), the accumulation ratio (R) can be computed for any dosage interval: R = 1/(1 – e–keτ), where τ is the dosage interval in hours. The accumulation ratio (R) is also equal to the ratio of the concentration at any time, t, after a single dose (CSD,t in mmol/L) and the steady-state concentration at that same time after the dose during multiple dosing (Css,t in mmol/L): R = Css,t/CSD,t or Css,t = R • CSD,t. Once a steady-state concentration can be computed for a dosage regimen, linear pharmacokinetic principles can be used to compute the dose required to achieve a target lithium steady-state serum concentration: Dnew = (Css,new/Css,old)Dold
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree