FIGURE 173-2 Scarlet fever exanthem. Finely punctate erythema has become confluent (scarlatiniform); petechiae can occur and have a linear configuration within the exanthem in body folds (Pastia’s lines). (From Fitzpatrick, Johnson, Wolff: Color Atlas and Synopsis of Clinical Dermatology, 4th ed, New York, McGraw-Hill, 2001, with permission.)
Skin and Soft Tissue Infections GAS—and occasionally other streptococcal species—can cause a variety of infections involving the skin, subcutaneous tissues, muscles, and fascia. While several clinical syndromes offer a useful means for classification of these infections, not all cases fit exactly into one category. The classic syndromes are general guides to predicting the level of tissue involvement in a particular patient, the probable clinical course, and the likelihood that surgical intervention or aggressive life support will be required.
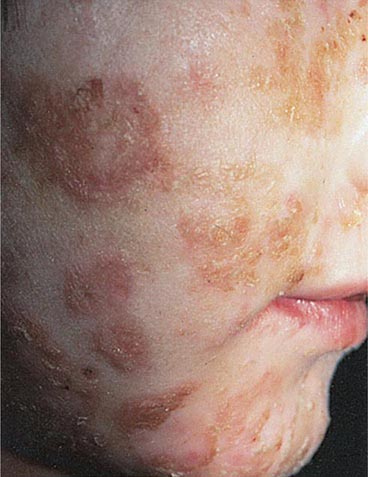
IMPETIGO (PYODERMA) Impetigo, a superficial infection of the skin, is caused primarily by GAS and occasionally by other streptococci or Staphylococcus aureus. Impetigo is seen most often in young children, tends to occur during warmer months, and is more common in semitropical or tropical climates than in cooler regions. Infection is more common among children living under conditions of poor hygiene. Prospective studies have shown that colonization of unbroken skin with GAS precedes clinical infection. Minor trauma, such as a scratch or an insect bite, may then serve to inoculate organisms into the skin. Impetigo is best prevented, therefore, by attention to adequate hygiene. The usual sites of involvement are the face (particularly around the nose and mouth) and the legs, although lesions may occur at other locations. Individual lesions begin as red papules, which evolve quickly into vesicular and then pustular lesions that break down and coalesce to form characteristic honeycomb-like crusts (Fig. 173-3). Lesions generally are not painful, and patients do not appear ill. Fever is not a feature of impetigo and, if present, suggests either infection extending to deeper tissues or another diagnosis. The classic presentation of impetigo usually poses little diagnostic difficulty. Cultures of impetiginous lesions often yield S. aureus as well as GAS. In almost all cases, streptococci are isolated initially and staphylococci appear later, presumably as secondary colonizing flora. In the past, penicillin was nearly always effective against these infections. However, an increasing frequency of penicillin treatment failure suggests that S. aureus may have become more prominent as a cause of impetigo. Bullous impetigo due to S. aureus is distinguished from typical streptococcal infection by more extensive, bullous lesions that break down and leave thin paper-like crusts instead of the thick amber crusts of streptococcal impetigo. Other skin lesions that may be confused with impetigo include herpetic lesions—either those of orolabial herpes simplex or those of chickenpox or zoster. Herpetic lesions can generally be distinguished by their appearance as more discrete, grouped vesicles and by a positive Tzanck test. In difficult cases, cultures of vesicular fluid should yield GAS in impetigo and the responsible virus in herpesvirus infections.
FIGURE 173-3 Impetigo contagiosa is a superficial streptococcal or Staphylococcus aureus infection consisting of honey-colored crusts and erythematous weeping erosions. Occasionally, bullous lesions may be seen. (Courtesy of Mary Spraker, MD; with permission.)
TREATMENT | STREPTOCOCCAL IMPETIGO |
Treatment of streptococcal impetigo is the same as that for streptococcal pharyngitis. In view of evidence that S. aureus has become a relatively frequent cause of impetigo, empirical regimens should cover both streptococci and S. aureus. For example, either dicloxacillin or cephalexin can be given at a dose of 250 mg four times daily for 10 days. Topical mupirocin ointment is also effective. Culture may be indicated to rule out methicillin-resistant S. aureus, especially if the response to empirical treatment is unsatisfactory. ARF is not a sequela to streptococcal skin infections, although PSGN may follow either skin or throat infection. The reason for this difference is not known. One hypothesis is that the immune response necessary for development of ARF occurs only after infection of the pharyngeal mucosa. In addition, the strains of GAS that cause pharyngitis are generally of different M protein types than those associated with skin infections; thus the strains that cause pharyngitis may have rheumatogenic potential, while the skin-infecting strains may not.
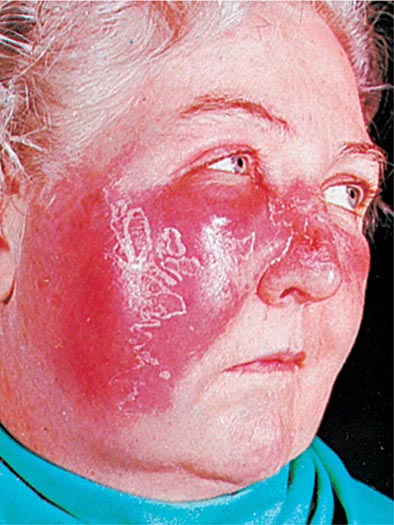
CELLULITIS Inoculation of organisms into the skin may lead to cellulitis: infection involving the skin and subcutaneous tissues. The portal of entry may be a traumatic or surgical wound, an insect bite, or any other break in skin integrity. Often, no entry site is apparent. One form of streptococcal cellulitis, erysipelas, is characterized by a bright red appearance of the involved skin, which forms a plateau sharply demarcated from surrounding normal skin (Fig. 173-4). The lesion is warm to the touch, may be tender, and appears shiny and swollen. The skin often has a peau d’orange texture, which is thought to reflect involvement of superficial lymphatics; superficial blebs or bullae may form, usually 2–3 days after onset. The lesion typically develops over a few hours and is associated with fever and chills. Erysipelas tends to occur on the malar area of the face (often with extension over the bridge of the nose to the contralateral malar region) and the lower extremities. After one episode, recurrence at the same site—sometimes years later—is not uncommon. Classic cases of erysipelas, with typical features, are almost always due to β-hemolytic streptococci, usually GAS and occasionally group C or G. Often, however, the appearance of streptococcal cellulitis is not sufficiently distinctive to permit a specific diagnosis on clinical grounds. The area involved may not be typical for erysipelas, the lesion may be less intensely red than usual and may fade into surrounding skin, and/or the patient may appear only mildly ill. In such cases, it is prudent to broaden the spectrum of empirical antimicrobial therapy to include other pathogens, particularly S. aureus, that can produce cellulitis with the same appearance. Staphylococcal infection should be suspected if cellulitis develops around a wound or an ulcer.
FIGURE 173-4 Erysipelas is a streptococcal infection of the superficial dermis and consists of well-demarcated, erythematous, edematous, warm plaques.
Streptococcal cellulitis tends to develop at anatomic sites in which normal lymphatic drainage has been disrupted, such as sites of prior cellulitis, the arm ipsilateral to a mastectomy and axillary lymph node dissection, a lower extremity previously involved in deep venous thrombosis or chronic lymphedema, or the leg from which a saphenous vein has been harvested for coronary artery bypass grafting. The organism may enter via a dermal breach some distance from the eventual site of clinical cellulitis. For example, some patients with recurrent leg cellulitis following saphenous vein removal stop having recurrent episodes only after treatment of tinea pedis on the affected extremity. Fissures in the skin presumably serve as a portal of entry for streptococci, which then produce infection more proximally in the leg at the site of previous injury. Streptococcal cellulitis may also involve recent surgical wounds. GAS is among the few bacterial pathogens that typically produce signs of wound infection and surrounding cellulitis within the first 24 h after surgery. These wound infections are usually associated with a thin exudate and may spread rapidly, either as cellulitis in the skin and subcutaneous tissue or as a deeper tissue infection (see below). Streptococcal wound infection or localized cellulitis may also be associated with lymphangitis, manifested by red streaks extending proximally along superficial lymphatics from the infection site.
TREATMENT | STREPTOCOCCAL CELLULITIS |
See Table 173-3 and Chap. 156.
DEEP SOFT-TISSUE INFECTIONS Necrotizing fasciitis (hemolytic streptococcal gangrene) involves the superficial and/or deep fascia investing the muscles of an extremity or the trunk. The source of the infection is either the skin, with organisms introduced into tissue through trauma (sometimes trivial), or the bowel flora, with organisms released during abdominal surgery or from an occult enteric source, such as a diverticular or appendiceal abscess. The inoculation site may be inapparent and is often some distance from the site of clinical involvement; e.g., the introduction of organisms via minor trauma to the hand may be associated with clinical infection of the tissues overlying the shoulder or chest. Cases associated with the bowel flora are usually polymicrobial, involving a mixture of anaerobic bacteria (such as Bacteroides fragilis or anaerobic streptococci) and facultative organisms (usually gram-negative bacilli). Cases unrelated to contamination from bowel organisms are most commonly caused by GAS alone or in combination with other organisms (most often S. aureus). Overall, GAS is implicated in ~60% of cases of necrotizing fasciitis. The onset of symptoms is usually quite acute and is marked by severe pain at the site of involvement, malaise, fever, chills, and a toxic appearance. The physical findings, particularly early on, may not be striking, with only minimal erythema of the overlying skin. Pain and tenderness are usually severe. In contrast, in more superficial cellulitis, the skin appearance is more abnormal, but pain and tenderness are only mild or moderate. As the infection progresses (often over several hours), the severity and extent of symptoms worsen, and skin changes become more evident, with the appearance of dusky or mottled erythema and edema. The marked tenderness of the involved area may evolve into anesthesia as the spreading inflammatory process produces infarction of cutaneous nerves.
Although myositis is more commonly due to S. aureus infection, GAS occasionally produces abscesses in skeletal muscles (streptococcal myositis), with little or no involvement of the surrounding fascia or overlying skin. The presentation is usually subacute, but a fulminant form has been described in association with severe systemic toxicity, bacteremia, and a high mortality rate. The fulminant form may reflect the same basic disease process seen in necrotizing fasciitis, but with the necrotizing inflammatory process extending into the muscles themselves rather than remaining limited to the fascial layers.
TREATMENT | DEEP SOFT-TISSUE INFECTIONS |
Once necrotizing fasciitis is suspected, early surgical exploration is both diagnostically and therapeutically indicated. Surgery reveals necrosis and inflammatory fluid tracking along the fascial planes above and between muscle groups, without involvement of the muscles themselves. The process usually extends beyond the area of clinical involvement, and extensive debridement is required. Drainage and debridement are central to the management of necrotizing fasciitis; antibiotic treatment is a useful adjunct (Table 173-3), but surgery is life-saving. Treatment for streptococcal myositis consists of surgical drainage—usually by an open procedure that permits evaluation of the extent of infection and ensures adequate debridement of involved tissues—and high-dose penicillin (Table 173-3).
Pneumonia and Empyema GAS is an occasional cause of pneumonia, generally in previously healthy individuals. The onset of symptoms may be abrupt or gradual. Pleuritic chest pain, fever, chills, and dyspnea are the characteristic manifestations. Cough is usually present but may not be prominent. Approximately one-half of patients with GAS pneumonia have an accompanying pleural effusion. In contrast to the sterile parapneumonic effusions typical of pneumococcal pneumonia, those complicating streptococcal pneumonia are almost always infected. The empyema fluid is usually visible by chest radiography on initial presentation, and its volume may increase rapidly. These pleural collections should be drained early, as they tend to become loculated rapidly, resulting in a chronic fibrotic reaction that may require thoracotomy for removal.
Bacteremia, Puerperal Sepsis, and Streptococcal Toxic Shock Syndrome GAS bacteremia is usually associated with an identifiable local infection. Bacteremia occurs rarely with otherwise uncomplicated pharyngitis, occasionally with cellulitis or pneumonia, and relatively frequently with necrotizing fasciitis. Bacteremia without an identified source raises the possibility of endocarditis, an occult abscess, or osteomyelitis. A variety of focal infections may arise secondarily from streptococcal bacteremia, including endocarditis, meningitis, septic arthritis, osteomyelitis, peritonitis, and visceral abscesses. GAS is occasionally implicated in infectious complications of childbirth, usually endometritis and associated bacteremia. In the preantibiotic era, puerperal sepsis was commonly caused by GAS; currently, it is more often caused by GBS. Several nosocomial outbreaks of puerperal GAS infection have been traced to an asymptomatic carrier, usually someone present at delivery. The site of carriage may be the skin, throat, anus, or vagina.
Beginning in the late 1980s, several reports described patients with GAS infections associated with shock and multisystem organ failure. This syndrome was called streptococcal TSS because it shares certain features with staphylococcal TSS. In 1993, a case definition for streptococcal TSS was formulated (Table 173-4). The general features of the illness include fever, hypotension, renal impairment, and respiratory distress syndrome. Various types of rash have been described, but rash usually does not develop. Laboratory abnormalities include a marked shift to the left in the white blood cell differential, with many immature granulocytes; hypocalcemia; hypoalbuminemia; and thrombocytopenia, which usually becomes more pronounced on the second or third day of illness. In contrast to patients with staphylococcal TSS, the majority with streptococcal TSS are bacteremic. The most common associated infection is a soft tissue infection—necrotizing fasciitis, myositis, or cellulitis—although a variety of other associated local infections have been described, including pneumonia, peritonitis, osteomyelitis, and myometritis. Streptococcal TSS is associated with a mortality rate of ≥30%, with most deaths secondary to shock and respiratory failure. Because of its rapidly progressive and lethal course, early recognition of the syndrome is essential. Patients should receive aggressive supportive care (fluid resuscitation, pressors, and mechanical ventilation) in addition to antimicrobial therapy and, in cases associated with necrotizing fasciitis, surgical debridement. Exactly why certain patients develop this fulminant syndrome is not known. Early studies of the streptococcal strains isolated from these patients demonstrated a strong association with the production of pyrogenic exotoxin A. This association has been inconsistent in subsequent case series. Pyrogenic exotoxin A and several other streptococcal exotoxins act as superantigens to trigger release of inflammatory cytokines from T lymphocytes. Fever, shock, and organ dysfunction in streptococcal TSS may reflect, in part, the systemic effects of superantigen-mediated cytokine release.
PROPOSED CASE DEFINITION FOR THE STREPTOCOCCAL TOXIC SHOCK SYNDROMEa |
aAn illness fulfilling criteria IA, IIA, and IIB is defined as a definite case. An illness fulfilling criteria IB, IIA, and IIB is defined as a probable case if no other etiology for the illness is identified.
Source: Modified from Working Group on Severe Streptococcal Infections: JAMA 269:390, 1993.
TREATMENT | STREPTOCOCCAL TOXIC SHOCK SYNDROME |
In light of the possible role of pyrogenic exotoxins or other streptococcal toxins in streptococcal TSS, treatment with clindamycin has been advocated by some authorities (Table 173-3), who argue that, through its direct action on protein synthesis, clindamycin is more effective in rapidly terminating toxin production than is penicillin—a cell-wall agent. Support for this view comes from studies of an experimental model of streptococcal myositis, in which mice given clindamycin had a higher rate of survival than those given penicillin. Comparable data on the treatment of human infections are not available, although retrospective analysis has suggested a better outcome when patients with invasive soft-tissue infection are treated with clindamycin rather than with cell wall-active antibiotics. Although clindamycin resistance in GAS is uncommon (<2% among U.S. isolates), it has been documented. Thus, if clindamycin is used for initial treatment of a critically ill patient, penicillin should be given as well until the antibiotic susceptibility of the streptococcal isolate is known. IV immunoglobulin has been used as adjunctive therapy for streptococcal TSS (Table 173-3). Pooled immunoglobulin preparations contain antibodies capable of neutralizing the effects of streptococcal toxins. Anecdotal reports and case series have suggested favorable clinical responses to IV immunoglobulin, but no adequately powered, prospective, controlled trials have been reported.
PREVENTION
No vaccine against GAS is commercially available. A formulation that consists of recombinant peptides containing epitopes of 26 M-protein types has undergone phase 1 and 2 testing in volunteers. Early results indicate that the vaccine is well tolerated and elicits type-specific antibody responses. Vaccines based on a conserved region of M protein or on a mixture of other conserved GAS protein antigens are in earlier stages of development.
Household contacts of individuals with invasive GAS infection (e.g., bacteremia, necrotizing fasciitis, or streptococcal TSS) are at greater risk of invasive infection than the general population. Asymptomatic pharyngeal colonization with GAS has been detected in up to 25% of persons with >4 h/d of same-room exposure to an index case. However, antibiotic prophylaxis is not routinely recommended for contacts of patients with invasive disease because such an approach (if effective) would require treatment of hundreds of contacts to prevent a single case.
STREPTOCOCCI OF GROUPS C AND G
Group C and group G streptococci are β-hemolytic bacteria that occasionally cause human infections similar to those caused by GAS. Strains that form small colonies on blood agar (<0.5 mm) are generally members of the Streptococcus milleri (Streptococcus intermedius, Streptococcus anginosus) group (see “Viridans Streptococci,” below). Large-colony group C and G streptococci of human origin are now considered a single species, Streptococcus dysgalactiae subspecies equisimilis. These organisms have been associated with pharyngitis, cellulitis and soft tissue infections, pneumonia, bacteremia, endocarditis, and septic arthritis. Puerperal sepsis, meningitis, epidural abscess, intraabdominal abscess, urinary tract infection, and neonatal sepsis have also been reported. Group C or G streptococcal bacteremia most often affects elderly or chronically ill patients and, in the absence of obvious local infection, is likely to reflect endocarditis. Septic arthritis, sometimes involving multiple joints, may complicate endocarditis or develop in its absence. Distinct streptococcal species of Lancefield group C cause infections in domesticated animals, especially horses and cattle; some human infections are acquired through contact with animals or consumption of unpasteurized milk. These zoonotic organisms include Streptococcus equi subspecies zooepidemicus and S. equi subspecies equi.
TREATMENT | GROUP C OR G STREPTOCOCCAL INFECTION |
Penicillin is the drug of choice for treatment of group C or G streptococcal infections. Antibiotic treatment is the same as for similar syndromes due to GAS (Table 173-3). Patients with bacteremia or septic arthritis should receive IV penicillin (2–4 mU every 4 h). All group C and G streptococci are sensitive to penicillin; nearly all are inhibited in vitro by concentrations of ≤0.03 μg/mL. Occasional isolates exhibit tolerance: although inhibited by low concentrations of penicillin, they are killed only by significantly higher concentrations. The clinical significance of tolerance is unknown. Because of the poor clinical response of some patients to penicillin alone, the addition of gentamicin (1 mg/kg every 8 h for patients with normal renal function) is recommended by some authorities for treatment of endocarditis or septic arthritis due to group C or G streptococci; however, combination therapy has not been shown to be superior to penicillin treatment alone. Patients with joint infections often require repeated aspiration or open drainage and debridement for cure; the response to treatment may be slow, particularly in debilitated patients and those with involvement of multiple joints. Infection of prosthetic joints almost always requires prosthesis removal in addition to antibiotic therapy.
GROUP B STREPTOCOCCI
Identified first as a cause of mastitis in cows, streptococci belonging to Lancefield’s group B have since been recognized as a major cause of sepsis and meningitis in human neonates. GBS is also a frequent cause of peripartum fever in women and an occasional cause of serious infection in nonpregnant adults. Since the widespread institution of prenatal screening for GBS in the 1990s, the incidence of neonatal infection per 1000 live births has fallen from ~2–3 cases to ~0.6 case. During the same period, GBS infection in adults with underlying chronic illnesses has become more common; adults now account for a larger proportion of invasive GBS infections than do newborns. Lancefield group B consists of a single species, S. agalactiae, which is definitively identified with specific antiserum to the group B cell wall–associated carbohydrate antigen. A streptococcal isolate can be classified presumptively as GBS on the basis of biochemical tests, including hydrolysis of sodium hippurate (in which 99% of isolates are positive), hydrolysis of bile esculin (in which 99–100% are negative), bacitracin susceptibility (in which 92% are resistant), and production of CAMP factor (in which 98–100% are positive). CAMP factor is a phospholipase produced by GBS that causes synergistic hemolysis with β lysin produced by certain strains of S. aureus. Its presence can be demonstrated by cross-streaking of the test isolate and an appropriate staphylococcal strain on a blood agar plate. GBS organisms causing human infections are encapsulated by one of ten antigenically distinct polysaccharides. The capsular polysaccharide is an important virulence factor. Antibodies to the capsular polysaccharide afford protection against GBS of the same (but not of a different) capsular type.
INFECTION IN NEONATES
Two general types of GBS infection in infants are defined by the age of the patient at presentation. Early-onset infections occur within the first week of life, with a median age of 20 h at onset. Approximately half of these infants have signs of GBS disease at birth. The infection is acquired during or shortly before birth from the colonized maternal genital tract. Surveillance studies have shown that 5–40% of women are vaginal or rectal carriers of GBS. Approximately 50% of infants delivered vaginally by carrier mothers become colonized, although only 1–2% develop clinically evident infection. Prematurity, prolonged labor, obstetric complications, and maternal fever are risk factors for early-onset infection. The presentation of early-onset infection is the same as that of other forms of neonatal sepsis. Typical findings include respiratory distress, lethargy, and hypotension. Essentially all infants with early-onset disease are bacteremic, one-third to one-half have pneumonia and/or respiratory distress syndrome, and one-third have meningitis.
Late-onset infections occur in infants 1 week to 3 months old and, in rare instances, in older infants (mean age at onset, 3–4 weeks). The infecting organism may be acquired during delivery (as in early-onset cases) or during later contact with a colonized mother, nursery personnel, or another source. Meningitis is the most common manifestation of late-onset infection and in most cases is associated with a strain of capsular type III. Infants present with fever, lethargy or irritability, poor feeding, and seizures. The various other types of late-onset infection include bacteremia without an identified source, osteomyelitis, septic arthritis, and facial cellulitis associated with submandibular or preauricular adenitis.
TREATMENT | GROUP B STREPTOCOCCAL INFECTION IN NEONATES |
Penicillin is the agent of choice for all GBS infections. Empirical broad-spectrum therapy for suspected bacterial sepsis, consisting of ampicillin and gentamicin, is generally administered until culture results become available. If cultures yield GBS, many pediatricians continue to administer gentamicin, along with ampicillin or penicillin, for a few days until clinical improvement becomes evident. Infants with bacteremia or soft tissue infection should receive penicillin at a dosage of 200,000 units/kg per day in divided doses. For meningitis, infants ≤7 days of age should receive 250,000–450,000 units/kg per day in three divided doses; infants >7 days of age should receive 450,000–500,000 units/kg per day in four divided doses. Meningitis should be treated for at least 14 days because of the risk of relapse with shorter courses.
PREVENTION
The incidence of GBS infection is unusually high among infants of women with risk factors: preterm delivery, early rupture of membranes (>24 h before delivery), prolonged labor, fever, or chorioamnionitis. Because the usual source of the organisms infecting a neonate is the mother’s birth canal, efforts have been made to prevent GBS infections by the identification of high-risk carrier mothers and their treatment with various forms of antibiotic prophylaxis or immunoprophylaxis. Prophylactic administration of ampicillin or penicillin to such patients during delivery reduces the risk of infection in the newborn. This approach has been hampered by logistical difficulties in identifying colonized women before delivery; the results of vaginal cultures early in pregnancy are poor predictors of carrier status at delivery. The CDC recommends screening for anogenital colonization at 35–37 weeks of pregnancy by a swab culture of the lower vagina and anorectum; intrapartum chemoprophylaxis is recommended for culture-positive women and for women who, regardless of culture status, have previously given birth to an infant with GBS infection or have a history of GBS bacteriuria during pregnancy. Women whose culture status is unknown and who develop premature labor (<37 weeks), prolonged rupture of membranes (>18 h), or intrapartum fever or who have a positive intrapartum nucleic acid amplification test for GBS should also receive intrapartum chemoprophylaxis. The recommended regimen for chemoprophylaxis is a loading dose of 5 million units of penicillin G followed by 2.5 million units every 4 h until delivery. Cefazolin is an alternative for women with a history of penicillin allergy who are thought not to be at high risk for anaphylaxis. For women with a history of immediate hypersensitivity, clindamycin may be substituted, but only if the colonizing isolate has been demonstrated to be susceptible. If susceptibility testing results are not available or indicate resistance, vancomycin should be used in this situation.
Treatment of all pregnant women who are colonized or have risk factors for neonatal infection will result in exposure of up to one-third of pregnant women and newborns to antibiotics, with the attendant risks of allergic reactions and selection for resistant organisms. Although still in the developmental stages, a GBS vaccine may ultimately offer a better solution to prevention. Because transplacental passage of maternal antibodies produces protective antibody levels in newborns, efforts are under way to develop a vaccine against GBS that can be given to childbearing-age women before or during pregnancy. Results of phase 1 clinical trials of GBS capsular polysaccharide–protein conjugate vaccines suggest that a multivalent conjugate vaccine would be safe and highly immunogenic.
INFECTION IN ADULTS
The majority of GBS infections in otherwise healthy adults are related to pregnancy and parturition. Peripartum fever, the most common manifestation, is sometimes accompanied by symptoms and signs of endometritis or chorioamnionitis (abdominal distention and uterine or adnexal tenderness). Blood and vaginal swab cultures are often positive. Bacteremia is usually transitory but occasionally results in meningitis or endocarditis. Infections in adults that are not associated with the peripartum period generally involve individuals who are elderly or have an underlying chronic illness, such as diabetes mellitus or a malignancy. Among the infections that develop with some frequency in adults are cellulitis and soft tissue infection (including infected diabetic skin ulcers), urinary tract infection, pneumonia, endocarditis, and septic arthritis. Other reported infections include meningitis, osteomyelitis, and intraabdominal or pelvic abscesses. Relapse or recurrence of invasive infection weeks to months after a first episode is documented in ~4% of cases.
TREATMENT | GROUP B STREPTOCOCCAL INFECTION IN ADULTS |
GBS is less sensitive to penicillin than GAS, requiring somewhat higher doses. Adults with serious localized infections (pneumonia, pyelonephritis, abscess) should receive doses of ~12 million units of penicillin G daily; patients with endocarditis or meningitis should receive 18–24 million units per day in divided doses. Vancomycin is an acceptable alternative for penicillin-allergic patients.
NONENTEROCOCCAL GROUP D STREPTOCOCCI
The main nonenterococcal group D streptococci that cause human infections were previously considered a single species, Streptococcus bovis. The organisms encompassed by S. bovis have been reclassified into two species, each of which has two subspecies: Streptococcus gallolyticus subspecies gallolyticus, S. gallolyticus subspecies pasteurianus, Streptococcus infantarius subspecies infantarius, and S. infantarius subspecies coli. Endocarditis caused by these organisms is often associated with neoplasms of the gastrointestinal tract—most frequently, a colon carcinoma or polyp—but is also reported in association with other bowel lesions. When occult gastrointestinal lesions are carefully sought, abnormalities are found in >60% of patients with endocarditis due to S. gallolyticus or S. infantarius. In contrast to the enterococci, nonenterococcal group D streptococci like these organisms are reliably killed by penicillin as a single agent, and penicillin is the agent of choice for the infections they cause.
VIRIDANS AND OTHER STREPTOCOCCI
VIRIDANS STREPTOCOCCI
Consisting of multiple species of α-hemolytic streptococci, the viridans streptococci are a heterogeneous group of organisms that are important agents of bacterial endocarditis (Chap. 155). Several species of viridans streptococci, including Streptococcus salivarius, Streptococcus mitis, Streptococcus sanguis, and Streptococcus mutans, are part of the normal flora of the mouth, where they live in close association with the teeth and gingiva. Some species contribute to the development of dental caries.
Previously known as Streptococcus morbillorum, Gemella morbillorum has been placed in a separate genus, along with Gemella haemolysans, on the basis of genetic-relatedness studies. These species resemble viridans streptococci with respect to habitat in the human host and associated infections.
The transient viridans streptococcal bacteremia induced by eating, toothbrushing, flossing, and other sources of minor trauma, together with adherence to biologic surfaces, is thought to account for the predilection of these organisms to cause endocarditis (see Fig. 155-1). Viridans streptococci are also isolated, often as part of a mixed flora, from sites of sinusitis, brain abscess, and liver abscess.
Viridans streptococcal bacteremia occurs relatively frequently in neutropenic patients, particularly after bone marrow transplantation or high-dose chemotherapy for cancer. Some of these patients develop a sepsis syndrome with high fever and shock. Risk factors for viridans streptococcal bacteremia include chemotherapy with high-dose cytosine arabinoside, prior treatment with trimethoprim-sulfamethoxazole or a fluoroquinolone, treatment with antacids or histamine antagonists, mucositis, and profound neutropenia.
The S. milleri group (also referred to as the S. intermedius or S. anginosus group) includes three species that cause human disease: S. intermedius, S. anginosus, and Streptococcus constellatus. These organisms are often considered viridans streptococci, although they differ somewhat from other viridans streptococci in both their hemolytic pattern (they may be α-, β-, or nonhemolytic) and the disease syndromes they cause. This group commonly produces suppurative infections, particularly abscesses of brain and abdominal viscera, and infections related to the oral cavity or respiratory tract, such as peritonsillar abscess, lung abscess, and empyema.
TREATMENT | INFECTION WITH VIRIDANS STREPTOCOCCI |
Isolates from neutropenic patients with bacteremia are often resistant to penicillin; thus these patients should be treated presumptively with vancomycin until the results of susceptibility testing become available. Viridans streptococci isolated in other clinical settings usually are sensitive to penicillin.
ABIOTROPHIA AND GRANULICATELLA SPECIES (NUTRITIONALLY VARIANT STREPTOCOCCI)
Occasional isolates cultured from the blood of patients with endocarditis fail to grow when subcultured on solid media. These nutritionally variant streptococci require supplemental thiol compounds or active forms of vitamin B6 (pyridoxal or pyridoxamine) for growth in the laboratory. The nutritionally variant streptococci are generally grouped with the viridans streptococci because they cause similar types of infections. However, they have been reclassified on the basis of 16S ribosomal RNA sequence comparisons into two separate genera: Abiotrophia, with a single species (Abiotrophia defectiva), and Granulicatella, with three species associated with human infection (Granulicatella adiacens, Granulicatella para-adiacens, and Granulicatella elegans).
TREATMENT | INFECTION WITH NUTRITIONALLY VARIANT STREPTOCOCCI |
Treatment failure and relapse appear to be more common in cases of endocarditis due to nutritionally variant streptococci than in those due to the usual viridans streptococci. Thus the addition of gentamicin (1 mg/kg every 8 h for patients with normal renal function) to the penicillin regimen is recommended for endocarditis due to the nutritionally variant organisms.
OTHER STREPTOCOCCI
Streptococcus suis is an important pathogen in swine and has been reported to cause meningitis in humans, usually in individuals with occupational exposure to pigs. Strains of S. suis associated with human infections have generally reacted with Lancefield group R typing serum and sometimes with group D typing serum as well. Isolates may be α- or β-hemolytic and are sensitive to penicillin. Streptococcus iniae, a pathogen of fish, has been associated with infections in humans who have handled live or freshly killed fish. Cellulitis of the hand is the most common form of human infection, although bacteremia and endocarditis have been reported. Anaerobic streptococci, or peptostreptococci, are part of the normal flora of the oral cavity, bowel, and vagina. Infections caused by the anaerobic streptococci are discussed in Chap. 201.
174 | Enterococcal Infections |
Enterococci have been recognized as potential human pathogens for more than a century, but only in recent years have these organisms acquired prominence as important causes of nosocomial infections. The ability of enterococci to survive and/or disseminate in the hospital environment and to acquire antibiotic resistance determinants makes the treatment of some enterococcal infections in critically ill patients a difficult challenge. Enterococci were first mentioned in the French literature in 1899; the “entérocoque” was found in the human gastrointestinal tract and was noted to have the potential to produce significant disease. Indeed, the first pathologic description of an enterococcal infection dates to the same year. A clinical isolate from a patient who died as a consequence of endocarditis was initially designated Micrococcus zymogenes, was later named Streptococcus faecalis subspecies zymogenes, and would now be classified as Enterococcus faecalis. The ability of this isolate to cause severe disease in both rabbits and mice illustrated its potential lethality in the appropriate settings.
ETIOLOGY
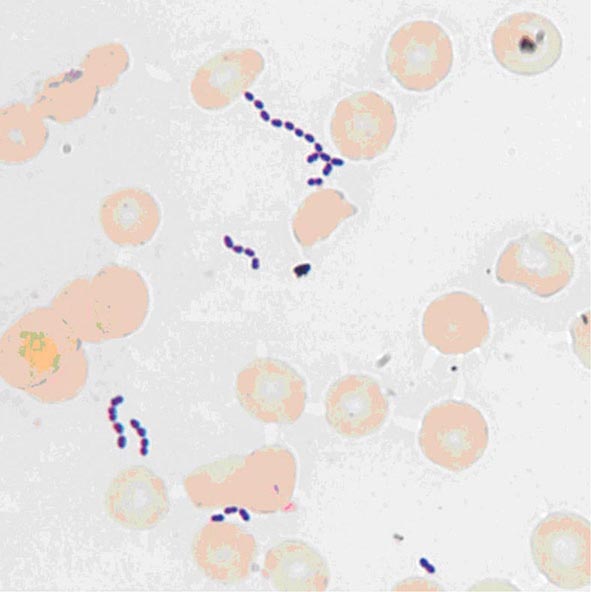
Enterococci are gram-positive organisms. In clinical specimens, they are usually observed as single cells, diplococci, or short chains (Fig. 174-1), although long chains are noted with some strains. Enterococci were originally classified as streptococci because organisms of the two genera share many morphologic and phenotypic characteristics, including a generally negative catalase reaction. Only DNA hybridization studies and later 16S rRNA sequencing clearly demonstrated that enterococci should be grouped as a genus distinct from the streptococci. Nonetheless, unlike the majority of streptococci, enterococci hydrolyze esculin in the presence of 40% bile salts and grow at high salt concentrations (e.g., 6.5%) and at high temperatures (46°C). Enterococci are usually reported by the clinical laboratory to be nonhemolytic on the basis of their inability to lyse the ovine or bovine red blood cells (RBCs) commonly used in agar plates; however, some strains of E. faecalis do lyse RBCs from humans, horses, and rabbits. The majority of clinically relevant enterococcal species hydrolyze pyrrolidonyl-β-naphthylamide (PYR); this characteristic is helpful in differentiating enterococci from organisms of the Streptococcus gallolyticus group (formerly known as S. bovis), which includes S. gallolyticus, Streptococcus pasteurianus, and Streptococcus infantarius, and from Leuconostoc species. Although at least 18 species of enterococci have been isolated from human infections, the overwhelming majority of cases are caused by two species, E. faecalis and Enterococcus faecium. Less frequently isolated species include Enterococcus gallinarum, Enterococcus durans, Enterococcus hirae, and Enterococcus avium.
FIGURE 174-1 Gram’s stain of cultured blood from a patient with enterococcal bacteremia. Oval gram-positive bacterial cells are arranged as diplococci and short chains. (Courtesy of Audrey Wanger, PhD.)
PATHOGENESIS
Enterococci are normal inhabitants of the large bowel of human adults, although they usually make up <1% of the culturable intestinal microflora. In the healthy human gastrointestinal tract, enterococci are typical symbionts that coexist with other gastrointestinal bacteria; in fact, the utility of certain enterococcal strains as probiotics in the treatment of diarrhea suggests their possible role in maintaining the homeostatic equilibrium of the bowel. Enterococci are intrinsically resistant to a variety of commonly used antibacterial drugs. One of the most important factors that disrupts this equilibrium and promotes increased gastrointestinal colonization by enterococci is the administration of antimicrobial agents. In particular, antibiotics that are excreted in the bile and have broad-spectrum activity (e.g., certain cephalosporins that target anaerobes and gram-negative bacteria) are usually associated with the recovery of higher numbers of enterococci from feces. This increased colonization appears to be due not only to the simple enterococcal replacement in a “biologic niche” after the eradication of competing components of the flora, but also (at least in mice) to the suppression—upon reduction of the gram-negative microflora by antibiotics—of important immunologic signals (e.g., by the lectin RegIIIγ) that help keep enterococcal counts low in the normal human bowel. Several studies have shown that higher levels of gastrointestinal colonization are a critical factor in the pathogenesis of enterococcal infections. However, the mechanisms by which enterococci successfully colonize the bowel and gain access to the lymphatics and/or bloodstream remain incompletely understood.
Several vertebrate, worm, and insect models have been developed to study the role of possible pathogenic determinants in both E. faecalis and E. faecium. Three main groups of virulence factors may increase the ability of enterococci to colonize the gastrointestinal tract and/or cause disease. The first group, enterococcal secreted factors, are molecules released outside the bacterial cell that contribute to the process of infection. The best-studied of these molecules include enterococcal hemolysin/cytolysin and two enterococcal proteases (gelatinase and serine protease). Enterococcal cytolysin is a heterodimeric toxin produced by some strains of E. faecalis that is capable of lysing human RBCs as well as polymorphonuclear leukocytes and macrophages. E. faecalis gelatinase and serine protease are thought to mediate virulence by several mechanisms, including the degradation of host tissues and the modification of critical components of the immune system. Mutants lacking the genes corresponding to these proteins are highly attenuated in experimental peritonitis, endocarditis, and endophthalmitis.
A second group of virulence factors, enterococcal surface components, are thought to contribute to bacterial attachment to extracellular matrix molecules in the human host. Several molecules on the surface of enterococci have been characterized and shown to play a role in the pathogenesis of enterococcal infections. Among the characterized adhesins is aggregation substance of E. faecalis, which mediates the attachment of bacterial cells to each other, thereby facilitating conjugative plasmid exchange. Several lines of evidence indicate that aggregation substance and enterococcal cytolysin act synergistically to increase the virulence potential of E. faecalis strains in experimental endocarditis. The surface protein adhesin of collagen of E. faecalis (Ace) and its E. faecium homologue (Acm) recognize adhesive matrix molecules (MSCRAMMs) involved in bacterial attachment to host proteins such as collagen, fibronectin, and fibrinogen; both Ace and Acm are important in the pathogenesis of experimental endocarditis. Pili of gram-positive bacteria have been shown to be important mediators of attachment to and invasion of host tissues and are considered potential targets for immunotherapy. Both E. faecalis and E. faecium have surface pili. Mutants of E. faecalis lacking pili are attenuated in biofilm production, experimental endocarditis, and urinary tract infections (UTIs). Other surface proteins that share structural homology with MSCRAMMs and appear to play a role in enterococcal attachment to the host and in virulence include the E. faecalis surface protein Esp and its E. faecium homologue Espfm, the second collagen adhesin of E. faecium (Scm), the surface proteins of E. faecium (Fms), SgrA (which binds to components of the basal lamina), and EcbA (which binds to collagen type V). Additional surface components apparently associated with pathogenicity include the Elr protein (a protein from the WxL family) and polysaccharides, which are thought to interfere with phagocytosis of the organism by host immune cells. Some E. faecalis strains appear to harbor at least three distinct classes of capsular polysaccharide; some of these polysaccharides play a role in virulence and are potential targets for immunotherapy.
The third group of virulence factors has not been well characterized but consists of the E. faecalis stress protein Gls24, which has been associated with enterococcal resistance to bile salts and appears to be important in the pathogenesis of endocarditis, and the hylEfm-containing plasmids of E. faecium, which are transferable between strains and increase gastrointestinal colonization by E. faecium. In mouse peritonitis, acquisition of these plasmids increased the lethality of a commensal strain of E. faecium. Recently, a gene encoding a regulator of oxidative stress (AsrR) has been identified as an important virulence factor of E. faecium.
![]() The ability to sequence bacterial genomes has increased our understanding of bacterial diversity, evolution, pathogenesis, and mechanisms of antibiotic resistance. The genome sequences of more than 560 enterococcal strains are currently available, and some have been entirely closed and annotated. Sequence analysis has shown that the genetic diversity of enterococci is related in large part to the acquisition of exogenous DNA and the mobilization of large chromosomal regions, resulting in recombination of the “core” genomes. In addition, analyses indicate that E. faecium harbors a malleable accessory genome incorporating a substantial content of exogenous elements, including DNA from phages. Indeed, a hospital-associated E. faecium clade that contains most clinical and outbreak-associated strains is the predominant genetic lineage circulating in hospitals around the world. This clade appears to be evolving rapidly, and genomic comparisons suggest that this lineage emerged 75 years ago—a time point that coincides with the introduction of antimicrobial drugs—and evolved from animal strains, not from human commensal isolates. An initial genomic separation within E. faecium appears to have occurred ~3000 years ago, simultaneous with urbanization and domestication of animals. This genomic information provides new clues with regard to the evolution of enterococci from commensal organisms to important nosocomial pathogens.
The ability to sequence bacterial genomes has increased our understanding of bacterial diversity, evolution, pathogenesis, and mechanisms of antibiotic resistance. The genome sequences of more than 560 enterococcal strains are currently available, and some have been entirely closed and annotated. Sequence analysis has shown that the genetic diversity of enterococci is related in large part to the acquisition of exogenous DNA and the mobilization of large chromosomal regions, resulting in recombination of the “core” genomes. In addition, analyses indicate that E. faecium harbors a malleable accessory genome incorporating a substantial content of exogenous elements, including DNA from phages. Indeed, a hospital-associated E. faecium clade that contains most clinical and outbreak-associated strains is the predominant genetic lineage circulating in hospitals around the world. This clade appears to be evolving rapidly, and genomic comparisons suggest that this lineage emerged 75 years ago—a time point that coincides with the introduction of antimicrobial drugs—and evolved from animal strains, not from human commensal isolates. An initial genomic separation within E. faecium appears to have occurred ~3000 years ago, simultaneous with urbanization and domestication of animals. This genomic information provides new clues with regard to the evolution of enterococci from commensal organisms to important nosocomial pathogens.
EPIDEMIOLOGY
According to the National Healthcare Safety Network of the Centers for Disease Control and Prevention, enterococci are the second most common organisms (after staphylococci) isolated from hospital-associated infections in the United States. Although E. faecalis remains the predominant species recovered from nosocomial infections, the isolation of E. faecium has increased substantially in the past 20 years. In fact, E. faecium is now almost as common as E. faecalis as an etiologic agent of hospital-associated infections. This point is important, because E. faecium is by far the most resistant and challenging enterococcal species to treat; indeed, more than 80% of E. faecium isolates recovered in U.S. hospitals are resistant to vancomycin, and more than 90% are resistant to ampicillin (historically the most effective β-lactam agent against enterococci). Resistance to vancomycin and ampicillin in E. faecalis isolates is much less common (~7% and ~4%, respectively).
The dynamics of enterococcal transmission and dissemination in the hospital environment have been extensively studied, with a focus on vancomycin-resistant enterococci (VRE). These studies have revealed that VRE colonization of the gastrointestinal tract is a critical step in the natural history of enterococcal disease and that a substantial proportion of patients colonized with VRE remain colonized for prolonged periods (sometimes >1 year) and are more likely to develop an Enterococcus-related illness (e.g., bacteremia). The most important factors associated with VRE colonization and persistence in the gut include prolonged hospitalization; long courses of antibiotic therapy; hospitalization in long-term-care facilities, surgical units, and/or intensive care units; organ transplantation; renal failure (particularly in patients undergoing hemodialysis) and/or diabetes; high Acute Physiology and Chronic Health Evaluation (APACHE) scores; and physical proximity to patients infected or colonized with VRE or these patients’ rooms. Once a patient becomes colonized with VRE, several key factors are involved in the organisms’ dissemination in the hospital environment. VRE can survive exposure to heat and certain disinfectants and have been found on numerous inanimate objects in the hospital, including bed rails, medical equipment, doorknobs, gloves, telephones, and computer keyboards. Thus health care workers and the environment play pivotal roles in enterococcal transmission from patient to patient, and infection control measures are crucial in breaking the chain of transmission. Moreover, two meta-analyses have found that, independent of the patient’s clinical status, VRE infection increases the risk of death over that among individuals infected with a glycopeptide-susceptible enterococcal strain.
![]() The epidemiology of enterococcal disease and the emergence of VRE have followed slightly different trends in other parts of the world than in the United States. In Europe, the emergence of VRE in the mid-1980s was seen primarily in isolates recovered from animals and healthy humans rather than from hospitalized patients. The presence of VRE was associated with the use of the glycopeptide avoparcin as a growth promoter in animal feeds; this association prompted the European Union to ban the use of this compound in animal husbandry in 1996. However, after an initial decrease in the isolation of VRE from animals and humans, the prevalence of hospital-associated VRE infections has slowly increased in certain European countries, with important regional differences. For example, rates of vancomycin resistance among E. faecium clinical isolates in Europe are highest in Greece, the United Kingdom, and Portugal (10–30%), whereas rates in the Scandinavian countries and the Netherlands are <1%. These regional differences have been attributed in part to the implementation of aggressive “search-and-destroy” policies of infection control in countries such as the Netherlands; these policies have kept the frequency of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) and VRE very low. Despite regional differences, rates of VRE continue to be much lower in Europe than in the United States. The reasons are not totally understood, although it has been postulated that this difference is related to the higher levels of human antibiotic use in the United States. Rates of enterococcal resistance to vancomycin in some Latin American countries are also lower (~4%) than those in the United States. Conversely, in Asia, rates of vancomycin resistance among enterococci appear to be similar to those in U.S. hospitals.
The epidemiology of enterococcal disease and the emergence of VRE have followed slightly different trends in other parts of the world than in the United States. In Europe, the emergence of VRE in the mid-1980s was seen primarily in isolates recovered from animals and healthy humans rather than from hospitalized patients. The presence of VRE was associated with the use of the glycopeptide avoparcin as a growth promoter in animal feeds; this association prompted the European Union to ban the use of this compound in animal husbandry in 1996. However, after an initial decrease in the isolation of VRE from animals and humans, the prevalence of hospital-associated VRE infections has slowly increased in certain European countries, with important regional differences. For example, rates of vancomycin resistance among E. faecium clinical isolates in Europe are highest in Greece, the United Kingdom, and Portugal (10–30%), whereas rates in the Scandinavian countries and the Netherlands are <1%. These regional differences have been attributed in part to the implementation of aggressive “search-and-destroy” policies of infection control in countries such as the Netherlands; these policies have kept the frequency of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) and VRE very low. Despite regional differences, rates of VRE continue to be much lower in Europe than in the United States. The reasons are not totally understood, although it has been postulated that this difference is related to the higher levels of human antibiotic use in the United States. Rates of enterococcal resistance to vancomycin in some Latin American countries are also lower (~4%) than those in the United States. Conversely, in Asia, rates of vancomycin resistance among enterococci appear to be similar to those in U.S. hospitals.
![]() As mentioned above, genomic analyses of vancomycin-resistant E. faecium in different parts of the world suggest that the emergence and dissemination of these organisms in the hospital environment worldwide are due to the success of a unique hospital-associated genetic clade that acquired the genes responsible for vancomycin resistance as well as other antibiotic resistance determinants.
As mentioned above, genomic analyses of vancomycin-resistant E. faecium in different parts of the world suggest that the emergence and dissemination of these organisms in the hospital environment worldwide are due to the success of a unique hospital-associated genetic clade that acquired the genes responsible for vancomycin resistance as well as other antibiotic resistance determinants.
CLINICAL SYNDROMES
URINARY TRACT INFECTION AND PROSTATITIS
Enterococci are well-known causes of nosocomial UTI—the most common infection caused by these organisms (Chap. 162). Enterococcal UTIs are usually associated with indwelling catheters, instrumentation, or anatomic abnormalities of the genitourinary tract, and it is often challenging to differentiate between true infection and colonization (particularly in patients with chronic indwelling catheters). The presence of leukocytes in the urine in conjunction with systemic manifestations (e.g., fever) or local signs and symptoms of infection with no other explanation and a positive urine culture (≥105 colony-forming units [CFU]/mL) suggests the diagnosis. Moreover, enterococcal UTIs often occur in critically ill patients whose comorbidities may obscure the diagnosis. In many cases, removal of the indwelling catheter may suffice to eradicate the organism without specific antimicrobial therapy. In rare circumstances, UTIs caused by enterococci may run a complicated course, with the development of pyelonephritis and perinephric abscesses that may be a portal of entry for bloodstream infections (see below). Enterococci are also known causes of chronic prostatitis, particularly in patients whose urinary tract has been manipulated surgically or endoscopically. These infections can be difficult to treat because the agents most potent against enterococci (i.e., aminopenicillins and glycopeptides) penetrate prostatic tissue poorly. Chronic prostatic infection can be a source of recurrent enterococcal bacteremia.
BACTEREMIA AND ENDOCARDITIS
Bacteremia without endocarditis is one of the most common presentations of enterococcal disease. Intravascular catheters and other devices are commonly associated with these bacteremic episodes (Chap. 168). Other well-known sources of enterococcal bacteremia include the gastrointestinal and hepatobiliary tracts; pelvic and intraabdominal foci; and, less frequently, wound infections, UTIs, and bone infections. In the United States, enterococci are ranked second (after coagulase-negative staphylococci) as etiologic agents of central line–associated bacteremia. Patients with enterococcal bacteremia usually have comorbidities and have been in the hospital for prolonged periods; they commonly have received several courses of antibiotics. Several studies indicate that the isolation of E. faecium from the blood may lead to worse outcomes and higher mortality rates than when other enterococcal species are isolated; this finding may be related to the higher prevalence of vancomycin and ampicillin resistance in E. faecium than in other enterococcal species, with the consequent reduction of therapeutic options. In many cases (usually when the gastrointestinal tract is the source), enterococcal bacteremia may be polymicrobial, with gram-negative organisms isolated at the same time. In addition, several cases have now been documented in which enterococcal bacteremia was associated with Strongyloides stercoralis hyperinfection syndrome in immunocompromised patients.
Enterococci are important causes of community- and health care–associated endocarditis, ranking second after staphylococci in the latter infections. The presumed initial source of bacteremia leading to endocarditis is the gastrointestinal or genitourinary tract—e.g., in patients who have malignant and inflammatory conditions of the gut or have undergone procedures in which these tracts are manipulated. The affected patients tend to be male and elderly and to have other debilitating diseases and heart conditions. Both prosthetic and native valves can be involved; mitral and aortic valves are affected most often. Community-associated endocarditis (usually caused by E. faecalis) also occurs in patients with no apparent risk factors or cardiac abnormalities. Endocarditis in women of childbearing age has been well described. The typical presentation of enterococcal endocarditis is a subacute course of fever, weight loss, malaise, and cardiac murmur; typical stigmata of endocarditis (e.g., petechiae, Osler’s nodes, Roth’s spots) are found in only a minority of patients. Atypical manifestations include arthralgias and manifestations of metastatic disease (splenic abscesses, hiccups, pain in the left flank, pleural effusion, and spondylodiscitis). Embolic complications are variable and can affect the brain. Heart failure is a common complication of enterococcal endocarditis, and valve replacement may be critical in curing this infection, particularly when multidrug-resistant organisms or major complications are involved. The duration of therapy is usually 4–6 weeks, with more prolonged courses suggested for multidrug-resistant isolates in the absence of valvular replacement.
MENINGITIS
Enterococcal meningitis is an uncommon disease (accounting for only ~4% of meningitis cases) that is usually associated with neurosurgical interventions and conditions such as shunts, central nervous system (CNS) trauma, and cerebrospinal fluid (CSF) leakage. In some instances—usually in patients with a debilitating condition, such as cardiovascular or congenital heart disease, chronic renal failure, malignancy, receipt of immunosuppressive therapy, or HIV/AIDS—presumed hematogenous seeding of the meninges is seen in infections such as endocarditis or bacteremia. Fever and changes in mental status are common, whereas overt meningeal signs are less so. CSF findings are consistent with bacterial infection—i.e., pleocytosis with a predominance of polymorphonuclear leukocytes (average, ~500/μL), an elevated serum protein level (usually >100 mg/dL), and a decreased glucose concentration (average, 28 mg/dL). Gram’s staining yields a positive result in about half of cases, with a high rate of organism recovery from CSF cultures; the most common species isolated are E. faecalis and E. faecium. Complications include hydrocephalus, brain abscesses, and stroke. As mentioned before for bacteremia, an association with Strongyloides hyperinfection has also been documented.
INTRAABDOMINAL, PELVIC, AND SOFT TISSUE INFECTIONS
As mentioned earlier, enterococci are part of the commensal flora of the gastrointestinal tract and can produce spontaneous peritonitis in cirrhotic individuals and in patients undergoing chronic ambulatory peritoneal dialysis (Chap. 159). These organisms are commonly found (usually along with other bacteria, including enteric gram-negative species and anaerobes) in clinical samples from intraabdominal and pelvic collections. The presence of enterococci in intraabdominal infections is sometimes considered to be of little clinical relevance. Several studies have shown that the role of enterococci in intraabdominal infections originating in the community and involving previously healthy patients is minor, because surgery and broad-spectrum antimicrobial drugs that do not target enterococci are often sufficient to treat these infections successfully. In the last few decades, however, these organisms have become prominent as a cause of intraabdominal infections in hospitalized patients because of the emergence and spread of vancomycin resistance among enterococci and the increase in rates of nosocomial infections due to multidrug-resistant E. faecium isolates. In fact, several studies have now documented treatment failures due to enterococci, with consequently increased rates of postoperative complications and death among patients with intraabdominal infections. Thus, anti-enterococcal therapy is recommended for nosocomial peritonitis in immunocompromised and severely ill patients who have had a prolonged hospital stay, have undergone multiple procedures, have persistent abdominal sepsis and collections, or have risk factors for the development of endocarditis (e.g., prosthetic or damaged heart valves). Conversely, specific treatment for enterococci in the first episode of intraabdominal infections originating in the community and affecting previously healthy patients with no important cardiac risk factors for endocarditis does not appear to be beneficial.
Enterococci are commonly isolated from soft tissue infections (Chap. 156), particularly those involving surgical wounds (Chap. 168). In fact, these organisms rank third as agents of nosocomial surgical-site infections, with E. faecalis the most frequently isolated species. The clinical relevance of enterococci in some of these infections—as in intraabdominal infections—is a matter of debate; differentiating between colonization and true infection is sometimes challenging, although in some cases enterococci have been recovered from lung, liver, and skin abscesses. Diabetic foot and decubitus ulcers are often colonized with enterococci and may be the portal of entry for bone infections.
OTHER INFECTIONS
Enterococci are well-known causes of neonatal infections, including sepsis (mostly late-onset), bacteremia, meningitis, pneumonia, and UTI. Outbreaks of enterococcal sepsis in neonatal units have been well documented. Risk factors for enterococcal disease in newborns include prematurity, low birth weight, indwelling devices, and abdominal surgery. Enterococci have also been described as etiologic agents of bone and joint infections, including vertebral osteomyelitis, usually in patients with underlying conditions such as diabetes or endocarditis. Similarly, enterococci have been isolated from bone infections in patients who have undergone arthroplasty or reconstruction of fractures with the placement of hardware. Because enterococci can produce a biofilm that is likely to alter the efficacy of otherwise active anti-enterococcal agents, treatment of infections that involve foreign material is challenging, and removal of the hardware may be necessary to eradicate the infection. Rare cases of enterococcal pneumonia, lung abscess, and spontaneous empyema have been described.
TREATMENT | ENTEROCOCCAL INFECTIONS |
GENERAL PRINCIPLES
Enterococci are intrinsically resistant and/or tolerant to several antimicrobial agents (with tolerance defined as lack of killing by drug concentrations 32 times higher than the minimal inhibitory concentration [MIC]). Monotherapy for endocarditis with a β-lactam antibiotic (to which many enterococci are tolerant) has produced disappointing results, with low cure rates at the end of therapy. However, the addition of an aminoglycoside to a cell wall–active agent (a β-lactam or a glycopeptide) increases cure rates and eradicates the organisms; moreover, this combination is synergistic and bactericidal in vitro. Therefore, for many decades, combination therapy with a cell wall–active agent and an aminoglycoside has been the standard of care for endovascular infections caused by enterococci. This synergistic effect can be explained, at least in part, by the increased penetration of the aminoglycoside into the bacterial cell, presumably as a result of cell wall alterations produced by the β-lactam (or glycopeptide). Nonetheless, attaining synergistic bactericidal activity in the treatment of severe enterococcal infections has become increasingly difficult because of the development of resistance to virtually all antibiotics available for this purpose.
The treatment of E. faecalis differs substantially from that of E. faecium (Tables 174-1 and 174-2), mainly because of differences in resistance profiles (see below). For example, resistance to ampicillin and vancomycin is rare in E. faecalis, whereas these antibiotics are only infrequently useful against current isolates of E. faecium. Moreover, as a consequence of the challenges and therapeutic limitations posed by the emergence of drug resistance in enterococci, valve replacement may need to be considered in the treatment of endocarditis caused by multidrug-resistant enterococci. Less severe infections are often related to indwelling intravascular catheters; removal of the catheter increases the likelihood of enterococcal eradication by a subsequent short course of appropriate antimicrobial therapy.
SUGGESTED REGIMENS FOR THE MANAGEMENT OF INFECTIONS CAUSED BY ENTEROCOCCUS FAECALIS |
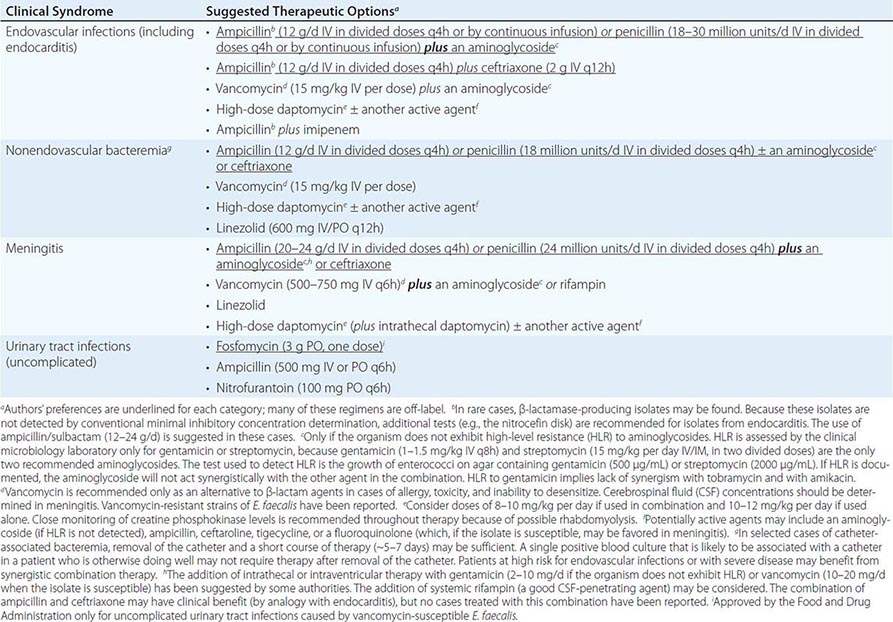
SUGGESTED REGIMENS FOR THE MANAGEMENT OF INFECTIONS CAUSED BY VANCOMYCIN- AND AMPICILLIN-RESISTANT ENTEROCOCCUS FAECIUM |
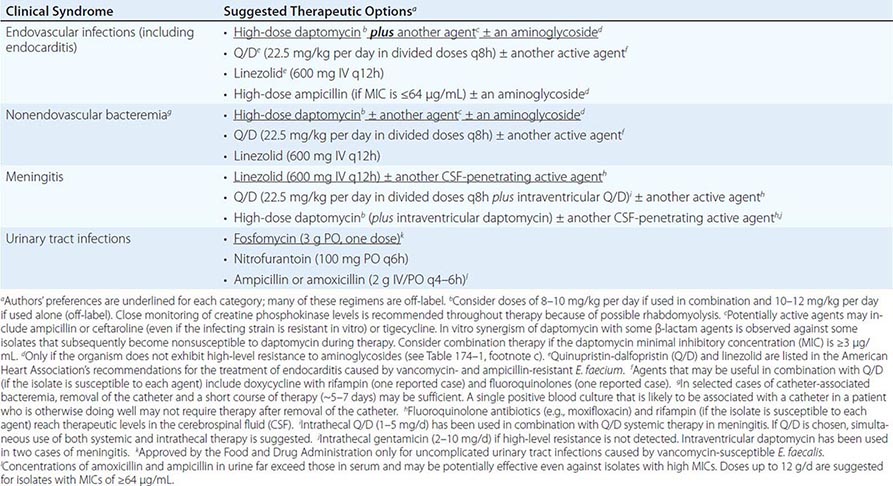
CHOICE OF ANTIMICROBIAL AGENTS
Among the β-lactams, the most active are the aminopenicillins (ampicillin, amoxicillin) and ureidopenicillins (i.e., piperacillin); next most active are penicillin G and imipenem. For E. faecium, a combination of high-dose ampicillin (up to 30 g/d) plus an aminoglycoside has been suggested—even for ampicillin-resistant strains if the MIC is ≤64 μg/mL—because a plasma ampicillin concentration of >100 μg/mL can be achieved at high doses. The only two aminoglycosides recommended for synergistic therapy in severe enterococcal infections are gentamicin and streptomycin. The use of amikacin is discouraged, tobramycin should never be used against E. faecium, and aminoglycoside monotherapy is not effective. Vancomycin is an alternative to β-lactam drugs for the treatment of E. faecalis infections but is less useful against E. faecium because resistance is common.
As mentioned above, use of the aminoglycoside–ampicillin combination for E. faecalis infections has become increasingly problematic because of toxicity in critically ill patients and increased rates of high-level resistance to aminoglycosides. A recent observational, nonrandomized, comparative study encompassing a multicenter cohort was conducted in 17 Spanish hospitals and 1 Italian hospital; this study found that the combination of ampicillin and ceftriaxone is as effective as ampicillin plus gentamicin in the treatment of E. faecalis endocarditis, with less risk of toxicity. Therefore, this regimen should be considered in patients at risk for aminoglycoside toxicity and could be considered for all patients.
Linezolid and quinupristin/dalfopristin (Q/D) are two agents approved by the U.S. Food and Drug Administration (FDA) for the treatment of some VRE infections (Table 174–2). Linezolid is not bactericidal, and its use in severe endovascular infections has produced mixed results; therefore, it is recommended only as an alternative to other agents. In addition, linezolid may cause significant toxicities (thrombocytopenia, peripheral neuropathy, and optic neuritis) when used in regimens given for >2 weeks. Nonetheless, linezolid may play a role in the treatment of enterococcal meningitis and other CNS infections, although clinical data are limited. Q/D is not active against most E. faecalis isolates, and its in vivo efficacy against E. faecium may often be compromised by resistance (see below). Adverse reactions to Q/D are common, including pain and inflammation at the infusion site and severe arthralgias and myalgias leading to discontinuation of treatment. Thus, Q/D should be used with caution and probably combined with other agents (Table 174–2).
The lipopeptide daptomycin is a bactericidal antibiotic with potent in vitro activity against all enterococci. Although daptomycin is not approved by the FDA for the treatment of VRE or E. faecium infections, it has been used alone (at high dosage) or in combination with other agents (ampicillin, ceftaroline, and tigecycline) with apparent success against multidrug-resistant enterococcal infections (Tables 174–1 and 174–2). The main adverse reactions to daptomycin are elevated creatine phosphokinase levels and eosinophilic pneumonitis (rare). Daptomycin is not useful against pulmonary infections because the pulmonary surfactant inhibits its antibacterial activity. Although the glycylcycline drug tigecycline is active in vitro against all enterococci (regardless of the isolates’ vancomycin susceptibility), its use as monotherapy for endovascular or severe enterococcal infections is not recommended because of low attainable blood levels. Telavancin, a lipoglycopeptide approved by the FDA for the treatment of skin and soft tissue infections as well as hospital-associated pneumonia, is active against vancomycin-susceptible enterococci but not VRE. Oritavancin, a compound of the same class that is active against VRE, has recently been approved by the FDA for the treatment of bacterial skin and soft tissue infections and may offer promise for the treatment of VRE in the future.
ANTIMICROBIAL RESISTANCE
As mentioned above, resistance to β-lactam agents continues to be observed only infrequently in E. faecalis, although rare outbreaks caused by β-lactamase-producing isolates have occurred in the United States and Argentina. However, ampicillin resistance is common in E. faecium. The mechanism of this resistance is related to a penicillin-binding protein (PBP) designated PBP5, which is the target of β-lactam antibiotics. PBP5 exhibits lower affinity for ampicillin and can synthesize cell wall in the presence of this antibiotic, even when other PBPs are inhibited. Two common mechanisms of high-level ampicillin resistance (MIC, >64 μg/mL) in clinical strains are (1) mutations in the PBP5-encoding gene that further decrease the protein’s affinity for ampicillin and (2) hyperproduction of PBP5. These factors preclude the use of all β-lactam agents in the treatment of E. faecium infections.
Vancomycin is a glycopeptide antibiotic that inhibits cell wall peptidoglycan synthesis in susceptible enterococci and has been widely used against enterococcal infections in clinical practice when the utility of β-lactams is limited by resistance, allergy, or adverse reactions. This effect is mediated by binding of the antibiotic to peptidoglycan precursors (UDP-MurNAc-pentapeptides) upon their exit from the bacterial cell cytoplasm. The interaction of vancomycin with the peptidoglycan is specific and involves the last two D-alanine residues of the precursor. The first isolates of VRE were documented in 1986, and vancomycin resistance (particularly in E. faecium) has since increased considerably around the world. The mechanism involves the replacement of the last D-alanine residue of peptidoglycan precursors with D-lactate or D-serine, with consequent high- and low-level resistance, respectively. There is significant heterogeneity among isolates, but either substitution substantially decreases the affinity of vancomycin for the peptidoglycan; with the D-lactate substitution, the MIC is increased by up to 1000-fold. Vancomycin-resistant organisms also produce enzymes that destroy the D-alanine-D-alanine ending precursors, ensuring that additional binding sites for vancomycin are not available.
High-level resistance to aminoglycosides (of which gentamicin and streptomycin are the only two tested by clinical laboratories) abolishes the synergism observed between cell wall–active agents and the aminoglycoside. This important phenotype is routinely sought in isolates from serious infections (Tables 174–1 and 174–2). The laboratory reports high-level resistance as gentamicin and streptomycin MICs of >500 μg/mL and >2000 μg/mL, respectively (agar dilution method) or as “SYN-R” (resistance to synergism). Genes encoding aminoglycoside-modifying enzymes are usually the cause of high-level resistance to these compounds and are widely disseminated among enterococci, decreasing the options for the treatment of severe enterococcal infections. The aforementioned enterococcal resistance to newer antibiotics such as linezolid (usually due to mutations in the 23S rRNA genes and the presence of an rRNA methylase), Q/D, daptomycin (involving major changes in cell membrane homeostasis), and tigecycline further reduces therapeutic alternatives.
175 | Diphtheria and Other Corynebacterial Infections |
DIPHTHERIA
Diphtheria is a nasopharyngeal and skin infection caused by Corynebacterium diphtheriae. Toxigenic strains of C. diphtheriae produce a protein toxin that causes systemic toxicity, myocarditis, and polyneuropathy. The toxin is associated with the formation of pseudomembranes in the pharynx during respiratory diphtheria. While toxigenic strains most frequently cause pharyngeal diphtheria, nontoxigenic strains commonly cause cutaneous disease.
ETIOLOGY
C. diphtheriae is a gram-positive bacillus that is unencapsulated, nonmotile, and nonsporulating. The organism was first identified microscopically in 1883 by Klebs and a year later was isolated in pure culture by Löffler in Robert Koch’s laboratory. The bacteria have a characteristic club-shaped bacillary appearance and typically form clusters of parallel rays, or palisades, that are referred to as “Chinese characters.” The specific laboratory media recommended for the cultivation of C. diphtheriae rely upon tellurite, colistin, or nalidixic acid for the organism’s selective isolation from other autochthonous pharyngeal microbes. C. diphtheriae may be isolated from individuals with both nontoxigenic (tox–) and toxigenic (tox+) phenotypes. Uchida and Pappenheimer demonstrated that corynebacteriophage beta carries the structural gene tox, which encodes diphtheria toxin, and that a family of closely related corynebacteriophages are responsible for toxigenic conversion of tox– C. diphtheriae to the tox+ phenotype. Moreover, lysogenic conversion from a nontoxigenic to a toxigenic phenotype has been shown to occur in situ. Growth of toxigenic strains of C. diphtheriae under iron-limiting conditions leads to the optimal expression of diphtheria toxin and is believed to be a pathogenic mechanism during human infection.
EPIDEMIOLOGY
![]() While in many regions diphtheria has been controlled in recent years with effective vaccination, there have been sporadic outbreaks in the United States and Europe. Diphtheria is still common in the Caribbean, Latin America, and the Indian subcontinent, where mass immunization programs are not enforced. Large-scale epidemics of diphtheria have occurred in the post-Soviet independent states. Additional outbreaks have been reported in Algeria, China, and Ecuador.
While in many regions diphtheria has been controlled in recent years with effective vaccination, there have been sporadic outbreaks in the United States and Europe. Diphtheria is still common in the Caribbean, Latin America, and the Indian subcontinent, where mass immunization programs are not enforced. Large-scale epidemics of diphtheria have occurred in the post-Soviet independent states. Additional outbreaks have been reported in Algeria, China, and Ecuador.
C. diphtheriae is transmitted via the aerosol route, usually during close contact with an infected person. There are no significant reservoirs other than humans. The incubation period for respiratory diphtheria is 2–5 days, but disease onset has occurred as late as 10 days after exposure. Prior to the vaccination era, most individuals over the age of 10 were immune to C. diphtheriae; infants were protected by maternal IgG antibodies but became susceptible after ~6 months of age. Thus, the disease primarily affected children and nonimmune young adults. In temperate regions, respiratory diphtheria occurs year-round but is most common during winter months.
The development of diphtheria antitoxin in 1898 by von Behring and of the diphtheria toxoid vaccine in 1924 by Ramon led to the near-elimination of diphtheria in Western countries. The annual incidence rate in the United States peaked in 1921 at 191 cases per 100,000 population. In contrast, since 1980, the annual figure in the United States has been <5 cases per 100,000. Nevertheless, pockets of colonization persist in North America, particularly in South Dakota, Ontario, and recently the state of Washington. Immunity to diphtheria induced by childhood vaccination gradually decreases in adulthood. An estimated 30% of men 60–69 years old have antitoxin titers below the protective level. In addition to older age and lack of vaccination, risk factors for diphtheria outbreaks include alcoholism, low socioeconomic status, crowded living conditions, and Native American ethnic background. An outbreak of diphtheria in Seattle, Washington, between 1972 and 1982 comprised 1100 cases, most of which were cutaneous. During the 1990s in the states of the former Soviet Union, a much larger diphtheria epidemic included more than 150,000 cases and more than 5000 deaths. Clonally related toxigenic C. diphtheriae strains of the ET8 complex were associated with this outbreak. Given that the ET8 complex expressed a toxin against which the prevalent diphtheria toxoid vaccine was effective, the epidemic was attributed to failure of the public health infrastructure to effectively vaccinate the population. Beginning in 1998, this epidemic was controlled by mass vaccination programs. During the epidemic, the incidence rate was high among individuals between 16 and 50 years of age. Socioeconomic instability, migration, deteriorating public health programs, frequent vaccine shortages, delayed implementation of vaccination and treatment in response to cases, and lack of public education and awareness were contributing factors.
Significant outbreaks of diphtheria and diphtheria-related mortality continue to be reported from many developing countries, particularly in Africa and Asia. Statistics collected by the World Health Organization indicated the occurrence of ~7000 reported diphtheria cases in 2008 and ~5000 diphtheria deaths in 2004. Although ~82% of the global population has been adequately vaccinated, only 26% of countries have successfully vaccinated >80% of individuals in all districts.
Cutaneous diphtheria is usually a secondary infection that follows a primary skin lesion due to trauma, allergy, or autoimmunity. Most often, these isolates lack the tox gene and thus do not express diphtheria toxin. In tropical latitudes, cutaneous diphtheria is more common than respiratory diphtheria. In contrast to respiratory disease, cutaneous diphtheria is not reportable in the United States. Nontoxigenic strains of C. diphtheriae have also been associated with pharyngitis in Europe, causing outbreaks among men who have sex with men and persons who use illicit IV drugs.
PATHOGENESIS AND IMMUNOLOGY
Diphtheria toxin produced by tox+ strains of C. diphtheriae is the primary virulence factor in clinical disease. The toxin is synthesized in precursor form; is released as a 535-amino-acid, single-chain protein; and, in sensitive species (e.g., guinea pigs and humans, but not mice or rats), has a 50% lethal dose of ~100 ng/kg of body weight. The toxin is produced in the pseudomembranous lesion and is taken up in the bloodstream, from which it is distributed to all organ systems in the body. Once bound to its cell surface receptor (a heparin-binding epidermal growth factor–like precursor), the toxin is internalized by receptor-mediated endocytosis and enters the cytosol from an acidified early endosomal compartment. In vitro, the toxin may be separated into two chains by digestion with serine proteases: the N-terminal A fragment and the C-terminal B fragment. Delivery of the A fragment into the eukaryotic cell cytosol results in irreversible inhibition of protein synthesis by NAD+-dependent ADP-ribosylation of elongation factor 2. The eventual result is the death of the cell.
In 1926, Ramon at the Institut Pasteur found that formalinization of diphtheria toxin resulted in the production of a nontoxic but highly immunogenic diphtheria toxoid. Subsequent studies showed that immunization with diphtheria toxoid elicited antibodies that neutralized the toxin and prevented most disease manifestations. In the 1930s, mass immunization of children and susceptible adults with diphtheria toxoid commenced in the United States and Europe.
Individuals with a diphtheria antitoxin titer of >0.01 U/mL are at low risk of disease. In populations where a majority of individuals have protective antitoxin titers, the carrier rate for toxigenic strains of C. diphtheriae decreases and the overall risk of diphtheria among susceptible individuals is reduced. Nevertheless, individuals with nonprotective titers may contract diphtheria through either travel or exposure to individuals who have recently returned from regions where the disease is endemic.
Characteristic pathologic findings of diphtheria include mucosal ulcers with a pseudomembranous coating composed of an inner band of fibrin and a luminal band of neutrophils. Initially white and firmly adherent, in advanced diphtheria the pseudomembranes turn gray or even green or black as necrosis progresses. Mucosal ulcers result from toxin-induced necrosis of the epithelium accompanied by edema, hyperemia, and vascular congestion of the submucosal base. A significant fibrinosuppurative exudate from the ulcer develops into the pseudomembrane. Ulcers and pseudomembranes in severe respiratory diphtheria may extend from the pharynx into medium-sized bronchial airways. Expanding and sloughing membranes may result in fatal airway obstruction.