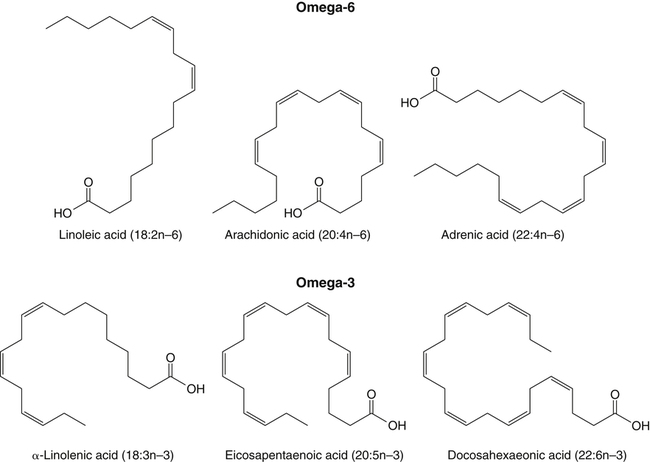
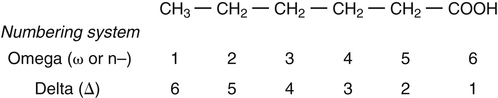
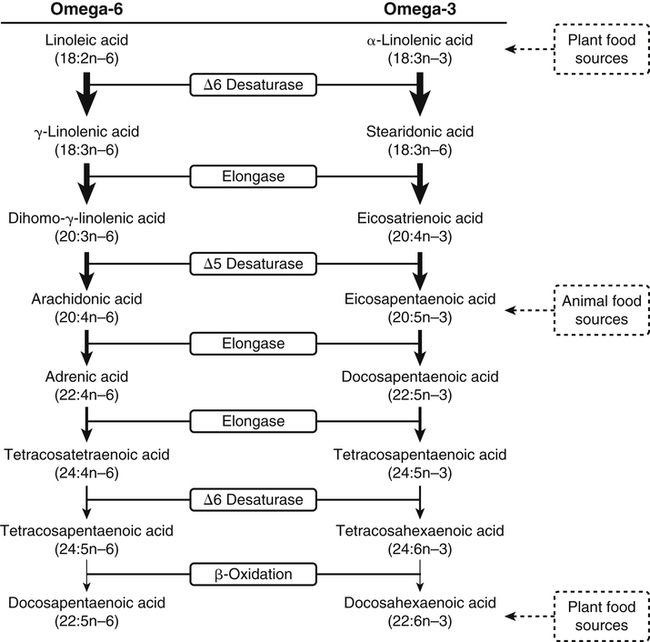
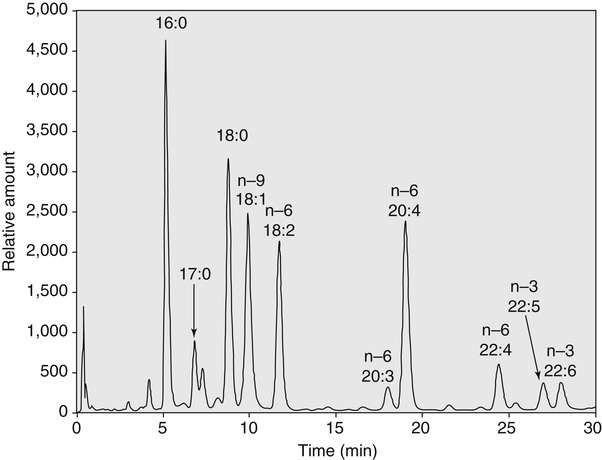
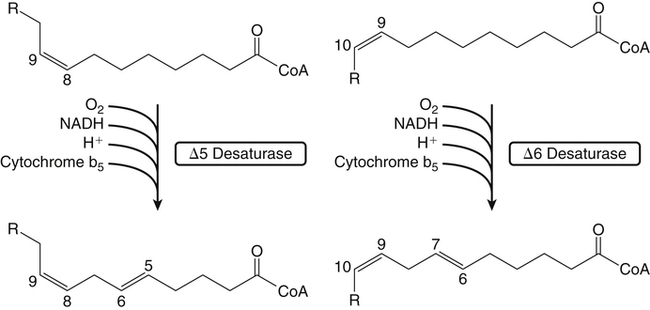
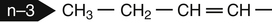Sarah K. Orr, BSc, Chuck T. Chen, BSc, Arthur A. Spector, MD and Richard P. Bazinet, PhD No well-defined disease occurred when experimental animals were fed a diet deficient in α-linolenic acid, the corresponding 18-carbon member of the n−3 PUFA class. Therefore it initially appeared that n−3 PUFAs were not essential nutrients and were present in the body simply because small amounts ordinarily are contained in the diet. This view gradually changed during the last 35 years because of increasing evidence that n−3 PUFAs are required for optimal visual and nervous system development (Innis, 2008). A consensus now exists that, like the n−6 class, the n−3 PUFAs are essential nutrients for humans. The PUFAs found in the body and in foods are mainly of the n−6 and n−3 classes. Figure 18-1 illustrates the chemical structures of the major n−6 and n−3 PUFAs present in humans and animals. The n−6 PUFAs are shown on the top and the n−3 PUFAs on the bottom. Although each class contains eight fatty acids, the six fatty acids shown in this figure account for more than 90% of the PUFAs present in the plasma and tissues under normal physiological conditions. The n−3 PUFAs are present in large amounts in the retina and certain areas of the brain. Like their n−6 counterparts, n−3 PUFAs can be structurally modified but cannot be synthesized completely in the body and ultimately must be obtained from the diet. The structures of the most important n−3 PUFAs are shown in Figure 18-1. α-Linolenic acid (18:3n−3), the 18-carbon member, is structurally similar to linoleic acid except for the presence of an additional double bond at C15. Some terrestrial plants synthesize small amounts of this fatty acid, and α-linolenic is present in soybean oil and canola oil. Larger amounts of α-linolenic acid are produced by vegetation that grows in cold water, and it is a prominent component in the food chain of fish and other marine animals. Although the intestinal mucosa can desaturate α-linolenic acid, most of the dietary intake is incorporated into the intestinal lipoproteins and absorbed by humans without structural modification. The terms highly unsaturated fatty acids, long-chain PUFAs, and very-long-chain PUFAs are sometimes used for PUFAs that contain four or more double bonds. These terms generally are applied to ARA (20:4n−6) and adrenic acid (22:4n−6) of the n−6 class and to EPA (20:5n−3) and DHA (22:6n−3) of the n−3 class (see Figure 18-1). The terms highly unsaturated fatty acids, long-chain PUFAs, and very-long-chain PUFAs were introduced to distinguish between the 20- and 22-carbon PUFAs, which produce most of the functional effects of essential fatty acids, and their 18-carbon precursors, which serve primarily as substrates for the synthesis of these more highly unsaturated derivatives. However, in chemistry the term long-chain fatty acid means any fatty acid greater than 12 carbons, thus leading to some confusion between the definitions of long-chain and very-long-chain fatty acids. Humans cannot completely synthesize either n−3 or n−6 PUFAs. However, all humans, even infants, can convert the 18-carbon members of each class to the corresponding 20- and 22-carbon products (Brenna et al., 2009). It is generally agreed that the human requirement for n−6 PUFAs can be fully satisfied by synthesis from dietary linoleic acid. However, there is ongoing debate as to whether humans, especially infants, can synthesize enough 20- and 22-carbon n−3 PUFAs from α-linolenic acid for optimal growth and development of the neural and visual systems. The synthesis of the longer, more highly unsaturated derivatives from the 18-carbon members of the n−3 and n−6 classes occurs through the pathway illustrated in Figure 18-2. Three types of reactions are involved: fatty acid chain elongation, desaturation, and β-oxidation (Sprecher, 2000). These reactions occur with both n−6 and n−3 PUFAs, but the two classes cannot be interconverted. Therefore an n−6 PUFA can be converted only to another n−6 PUFA, and likewise, an n−3 PUFA can be converted only to another n−3 PUFA. Therefore both classes of essential fatty acids are necessary in the diet. Of related interest, a gene from Caenorhabditis elegans encoding an n−3 desaturase, capable of converting n−6 PUFAs into n−3 PUFAs, has been isolated and transfected into mice and pigs, allowing them to synthesize n−3 PUFAs from n−6 PUFAs (Kang et al., 2004; Lai et al., 2006). Fatty acids are elongated in the endoplasmic reticulum (ER) through the mechanism illustrated in Figure 18-3. The fatty acid must be in the form of an acyl-CoA, and malonyl-CoA is the elongating agent. In the condensation reaction, which is the rate-limiting step, the free carboxyl group of malonyl-CoA is released as CO2 and the remaining 2-carbon fragment is attached to the fatty acid carbonyl group by displacement of CoA. Finally, the carbonyl group, which is C3 in the elongated product, is reduced in a three-step process that utilizes two NADPH molecules. The position of the double bonds does not shift relative to the methyl end when a PUFA is elongated, and their numbering remains the same in the n− or omega nomenclature. However, the numbering of the double bonds changes in the Δ nomenclature because the 2-carbon fragment that adds becomes C1 and C2 of the lengthened product. Therefore when 6,9,12-18:3 undergoes one elongation, the resulting 20-carbon fatty acid is 8,11,14-20:3. A fatty acid can undergo more than one elongation. Each elongation sequence consists of the enzymatic reactions shown in Figure 18-3 and uses two NADPH, and the fatty acid is lengthened by the addition of two carbons to the carboxyl end. All the elongation enzymes that have been studied effectively utilize both n−3 and n−6 PUFAs. However, there are at least five different human long-chain fatty acid elongase genes, denoted ELOVL1 to ELOVL5 (Jakobsson et al., 2006). The expression of these genes is tissue dependent. Furthermore, each ELOVL enzyme has different substrate specificity, although there is some overlap. For example, ELOVL5 acts on 18- and 20-carbon fatty acids, whereas ELOVL2 and ELOVL4 act on 20- and 22-carbon fatty acids. Consequently, at least two different fatty acid elongation enzymes operating in sequence are needed to convert an 18-carbon polyunsaturated fatty acid to the 24-carbon intermediate, and the enzymes that act in one tissue may be different from those that act in another tissue. These factors make elongation a complicated process that still is not fully understood. Although several genes may encode the FADS enzymes, in terms of PUFA metabolism, FADS1 and FADS2 are the most studied. FADS1 is the fatty acid Δ5-desaturase, and FADS2 is the fatty acid Δ6-desaturase. The genes coding for FADS1 and FADS2 are located on human chromosome 11q12-q13.1 in reverse orientation, separated by about 10,000 bp (Marquardt et al., 2000). The expression of these two genes is coordinately regulated. In addition, a third desaturase gene, FADS3, is located in the 11q12-q13.1 region, but the function of its gene product is unknown (Lattka et al., 2010). Figure 18-2 illustrates where the fatty acid Δ5- and Δ6-desaturases act in essential fatty acid metabolism. Both fatty acid desaturases can utilize either n−3 or n−6 polyunsaturated fatty acyl-CoA substrates, and they both require O2, NADH, cytochrome b5, and cytochrome b5 reductase. Figure 18-4 illustrates the two reactions. The desaturases act on the segment of the acyl-CoA chain between the carboxyl group and the first existing double bond. The Δ5-desaturase acts on polyunsaturated acyl-CoAs that have the first double bond at C8, inserting the new double bond at C5. This enzyme acts at only one point in the metabolic pathway, converting 20:3n−6 to ARA in n−6 PUFA metabolism and 20:4n−3 to EPA in n−3 PUFA metabolism. The Δ6-desaturase acts on polyunsaturated fatty acyl-CoA substrates that have the first double bond at C9, and inserts the new double bond at C6. There is only one fatty acid Δ6-desaturase, and this enzyme functions twice in n−3 PUFA metabolism, converting α-linolenic acid to 18:4n−3 and 24:5n−3 to 24:6n−3 (Sprecher, 2000). The Δ6-desaturase ordinarily functions only once in n−6 PUFA metabolism, converting linoleic acid to 18:3n−6. It also is capable of converting 24:4n−6 to 24:5n−6, but this occurs to an appreciable extent only if there is a deficiency of n−3 PUFAs. Conversion of the 24-carbon acyl-CoA intermediates to the 22-carbon end products is thought to occur through peroxisomal fatty acid oxidation, a β-oxidation system that shortens very-long-chain fatty acids. This process requires transport of the 24-carbon intermediate from the ER to the peroxisomes and, subsequently, transport of the 22-carbon product back to the ER where it is incorporated into tissue lipids. As shown in Figure 18-5, the retroconversion reaction requires O2, FAD, NAD+, and CoA, and it removes two carbons in the form of acetyl-CoA from the carboxyl end of the fatty acyl-CoA. The peroxisomal enzymes that catalyze this β-oxidation process are straight-chain acyl-CoA oxidase, D-bifunctional protein, and either 3-ketoacyl-CoA thiolase or sterol carrier protein X (SCP-X) (Ferdinandusse et al., 2001). In n−3 PUFA metabolism, this process converts 24:6n−3 to DHA. The numbering of the carbons in the Δ nomenclature changes when retroconversion occurs because the carbons that were numbered 1 and 2 in the original fatty acid are removed. Therefore the C6 double bond in the 24-carbon intermediate becomes the C4 double bond of DHA, the 22-carbon product. A similar process can occur with n−6 PUFAs to produce 22:5n−6 from 24:5n−6 (see Figure 18-2). Retroconversion also appears to be responsible for the increase in C20 PUFAs when C22 PUFAs are fed (e.g., increase in arachidonate when 22:4n−6 is fed, or of EPA when 22:5n−3 is fed). Thus elongation, desaturation, and retroconversion together may enable the body to utilize whichever n−3 and n−6 PUFAs are available in the diet to produce all of the necessary members of these essential fatty acid classes. Both dietary intake and metabolism influence the types of PUFAs that accumulate in the body. Western diets typically contain about 10 times more n−6 than n−3 PUFAs. Linoleic acid is the most abundant PUFA in the diet. Dietary PUFAs are incorporated into the lipids in chylomicrons produced by the small intestinal absorptive cells, and these lipoproteins are a major source of essential fatty acids for the tissues in the postprandial state. Many tissues are able to convert linoleic acid to ARA through the pathway illustrated in Figure 18-2, and linoleic acid (18:2n−6) and ARA (20:4n−6) are the main n−6 PUFAs that accumulate in the body. Very little α-linolenic acid (18:3n−3) ordinarily is present in the plasma or tissues, and unless the diet is supplemented with fish oil or n−3 PUFA ethyl esters, there also is little EPA (20:5n−3). The levels of PUFAs present in the plasma lipids of human subjects who consumed western diets are shown in Table 18-1 (Edelstein, 1986). These data show that n−6 PUFAs accounted for 17% of the fatty acids in the plasma free fatty acid fraction, 37% of the fatty acids in phospholipids, 22% of the fatty acids in triacylglycerols, and 59% of the fatty acids in cholesteryl esters. Linoleic acid and ARA comprised most of the n−6 PUFAs contained in these plasma lipids. In contrast to the high n−6 PUFA content, n−3 PUFAs comprised only 1% to 3% of the total fatty acids in any of the plasma lipid fractions. TABLE 18-1 Essential Fatty Acid Composition of Normal Human Plasma Lipids ∗Abbreviated as ratio of number of carbons to number of double bonds. †Phospholipids contain 0.65 ± 0.08% 20:5n−3 and 0.77 ± 0.03% 22:5n−3. The other lipid fractions contain only trace amounts (<0.3%) of these n−3 fatty acids. ‡Cholesteryl esters contain 1.07 ± 0.07% 18:3n−6, but the other lipid fractions contain only trace amounts. §The lipids contain only trace amounts (<0.5%) of 22:4n−6 and 22:5n−6. Modified from data compiled by Edelstein, C. (1986). General properties of plasma lipoproteins and apoproteins. In A. M. Scanu & A. A. Spector (Eds.), Biochemistry and biology of the plasma lipoproteins (pp. 495–505). New York: Marcel Dekker. Figure 18-6 shows the fatty acid composition of normal human erythrocytes from a person consuming a typical western diet, as determined by gas-liquid chromatography. Many more n−6 than n−3 PUFAs are contained in the erythrocyte lipids. The n−6 PUFAs present are 18:2n−6, 20:3n−6, 20:4n−6, and 22:4n−6, with linoleic acid (18:2n−6) and ARA (20:4n−6) accounting for about 80% of the total. The small amount of n−3 PUFAs are distributed almost equally between 22:5n−3 and DHA (22:6n−3).
Lipid Metabolism
Polyunsaturated Fatty Acids
Discovery of Essential Fatty Acids
Structure of Polyunsaturated Fatty Acids
The n−3 Polyunsaturated Fatty Acids
Highly Unsaturated Fatty Acids, Long-Chain Pufas, and Very-Long-Chain Pufas
Essential Fatty Acid Metabolism
Synthesis of 20- And 22-Carbon Pufas
Fatty Acid Elongation
Fatty Acid Desaturation
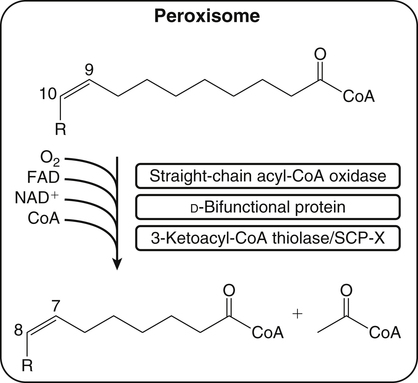
Retroconversion in the Peroxisomes
Essential Fatty Acid Composition of Plasma and Tissue Lipids
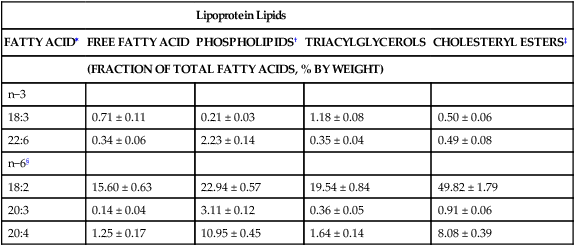
Lipoprotein Lipids
FATTY ACID∗
FREE FATTY ACID
PHOSPHOLIPIDS†
TRIACYLGLYCEROLS
CHOLESTERYL ESTERS‡
(FRACTION OF TOTAL FATTY ACIDS, % BY WEIGHT)
n−3
18:3
0.71 ± 0.11
0.21 ± 0.03
1.18 ± 0.08
0.50 ± 0.06
22:6
0.34 ± 0.06
2.23 ± 0.14
0.35 ± 0.04
0.49 ± 0.08
n−6§
18:2
15.60 ± 0.63
22.94 ± 0.57
19.54 ± 0.84
49.82 ± 1.79
20:3
0.14 ± 0.04
3.11 ± 0.12
0.36 ± 0.05
0.91 ± 0.06
20:4
1.25 ± 0.17
10.95 ± 0.45
1.64 ± 0.14
8.08 ± 0.39
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine