INTRODUCTION
LEARNING OBJECTIVES
After studying this chapter you should understand:
The factors that determine leukocyte counts in the peripheral blood.
The major causes of granulocytosis (neutrophilia) and other forms of leukocytosis.
The causes and consequences of leukopenia.
The mechanisms underlying qualitative disorders of neutrophil function.
The causes and consequences of hemophagocytic lymphohistiocytosis (macrophage activation syndrome).
Leukocytes constitute the cellular components of the innate and adaptive immune system and are critical for host defense. These cells mediate acute and chronic inflammation, modulate immune responses, and protect the host against numerous pathogens. It is no surprise that defects in leukocyte function predispose to various kinds of infection in proportion to the nature and severity of the defect.
Disorders affecting leukocytes can be divided broadly into malignant disorders (tumors of leukocytes or their progenitors) and non-malignant disorders. The malignant disorders are uncommon but clinically important entities that are covered in subsequent chapters. Here we consider the much more common non-malignant abnormalities that affect leukocytes. These can involve any of the leukocytes (neutrophils, eosinophils, basophils, monocytes, B cells, T cells, and natural killer cells), but the disorders of greatest clinical relevance affect neutrophils; these will be our major focus.
OVERVIEW OF NEUTROPHIL DISORDERS
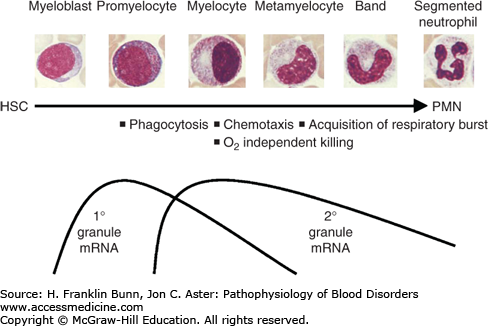
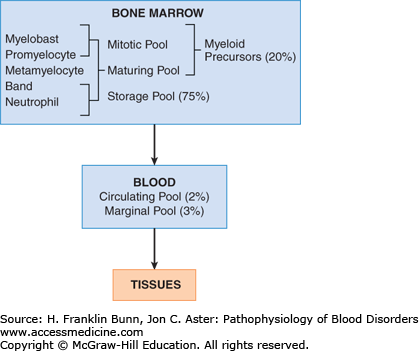
Like other formed elements of the peripheral blood, neutrophils are the progeny of earlier progenitors that acquire the capacity to function as they mature in the bone marrow (Fig. 18-1). Neutrophils are distributed within the body in several discrete and highly dynamic compartments or pools (Fig. 18-2). In the marrow, mitotically active myeloid progenitors mature over a period of 7 to 10 days to give rise to large numbers of neutrophils. Under steady-state conditions, most of these newly formed neutrophils die without ever being released into the blood, constituting a storage pool that can be called upon during times of increased need. Upon release from the marrow, neutrophils enter the circulating pool, the only pool that is measured clinically. On average, neutrophils circulate for only 3 to 6 hours before migrating into tissues, where they may survive for up to 3 days. At any given time, roughly half of the neutrophils in the blood are adherent to vessel walls; these cells are referred to as the marginal pool. Finally, some circulating neutrophils are sequestered in the spleen. Normally, <5% of the neutrophils in the body are in the circulating pool, which turns over quickly. As a result, stimuli that alter the release of neutrophils from the marrow, neutrophil margination, or the migration of neutrophils into tissues can have rapid and sometimes profound effects on the peripheral blood neutrophil count.
FIGURE 18-1
Myelopoiesis. As granulocytes develop and mature, they go through a series of recognizable morphologic stages that correlate with the expression of genes that confer the specific functions indicated on the time line. HSC: hematopoietic stem cell (Used with permission from Gaines P and Berliner N. Granulocytopoiesis. In Clinical Hematology, Young N, ed, Philadelphia, USA, Mosby/Elsevier, 2006, 62.)
In most clinical laboratories, the normal white blood cell count ranges from 4500 to 11,000/μL, with approximately 60% of the cells being neutrophils. Leukocytosis, an elevation in the white blood cell count, usually stems from a pathologic insult that stimulates a systemic inflammatory reaction. By far the most common form of leukocytosis is granulocytosis, an increase in neutrophils also known as neutrophilia.
Acute inflammation of any cause can produce the rapid appearance of granulocytosis by mobilizing neutrophils from the marrow storage pool. In particularly severe bacterial infections, immature granulocytic forms, such as band cells or even earlier progenitors, may be released into the blood (a finding also called a left-shift), and in extreme cases the white blood cell count may rise to >50,000/mm3. In such instances the marked leukocytosis and the presence of immature cells may mimic the appearance of a myeloid leukemia (leukemoid reaction). Occasionally, the inflammatory process may involve the marrow itself and produce marrow fibrosis and distortion, leading not only to the release of immature myeloid elements but also nucleated erythroid progenitors and misshapen tear-drop red cells. This combination of features in a peripheral smear is referred to as leukoerythroblastosis. If the marrow involvement is extensive, myelophthisic anemia may result (Chapter 4). It should be emphasized, however, that in the vast majority of cases granulocytosis is a reaction to disorders involving tissues other than the marrow. In contrast, neutropenia, an abnormally low neutrophil count, is often caused by defects in neutrophil production that may require a bone marrow examination for evaluation.
Primary disorders associated with neutrophil dysfunction usually have a genetic basis. Although rare, they are of interest because unraveling of their pathogenesis has led to the identification of genes that regulate normal neutrophil development and function.
GRANULOCYTOSIS
Granulocytosis can be primary or secondary (Table 18-1). Primary disorders associated with granulocytosis include myeloid neoplasms (discussed in subsequent chapters) and a variety of genetic disorders that merit brief mention.
Hereditary neutrophilia is a rare form of chronic granulocytosis that usually exhibits an autosomal dominant pattern of inheritance. In some families it is caused by gain-of-function mutations in the granulocyte colonystimulating factor (G-CSF) receptor that lead to marrow hyperstimulation and increased neutrophil production.
Down syndrome (trisomy 21) is the most common constitutional chromosomal disorder. About 10% of infants with Down syndrome develop a transient myeloproliferative disorder characterized by granulocytosis, circulating immature myeloid precursors, and hepatosplenomegaly. This disorder appears to be caused by acquired mutations in the transcription factor GATA-1 that result in deregulated growth of myeloid progenitors. In approximately 75% of patients, the disorder resolves spontaneously, but the remaining patients develop full-blown acute myeloid leukemia over a period of months to several years.
Leukocyte adhesion deficiency (LAD) is a rare autosomal recessive disorder that is described later under functional disorders of neutrophils.
Secondary causes of granulocytosis are much more common than primary causes and can be sorted into the following pathogenic categories.
Stress. Acute stress is associated with elevated levels of catecholamines and glucocorticoids, both of which cause rapid demargination of neutrophils. Psychic and physical stress, exercise, seizures, and pain can all cause neutrophilia through this mechanism.
Infection. Early in infection, factors derived from inflammatory cells such as interleukin-1 and tumor necrosis factor increase the release of neutrophils from the marrow storage pool. When an inflammatory stimulus is sustained, a variety of cells including T cells and macrophages release growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF. These factors enhance the growth and survival of granulocyte progenitors in the marrow and, over a period of 5 to 7 days, increase the rate of neutrophil production. In some instances (eg, disseminated tuberculosis) chronic infections involve the marrow directly and produce a leukoerythroblastic picture in the peripheral blood, owing to marrow inflammation and fibrosis.
Inflammation of noninfectious etiology can produce neutrophilia through the same mechanisms as those cited under infection.
Drug reactions can produce neutrophilia through various mechanisms. β-Adrenergic drugs and glucocorticoids cause neutrophils to demarginate, and glucocorticoids also cause a modest increase in the release of neutrophils from the marrow storage pool. Anabolic steroids such as testosterone stimulate hematopoiesis and may cause mild granulocytosis. Lithium used in the treatment of bipolar disorder can cause granulocytosis by stimulating the release of colony-stimulating factors from marrow stromal cells.
Marrow hyperstimulation. Granulocytosis stemming from elevated levels of hematopoietic growth factors and increased marrow production of neutrophils may be seen in hemolytic anemias and immune thrombocytopenic purpura or following treatment of megaloblastic anemias with folate or vitamin B12. On occasion, cancers produce hematopoietic growth factors such as G-CSF that lead to paraneoplastic neutrophilia.
Splenectomy. The asplenic state may be associated with mild granulocytosis due to decreased sequestration of neutrophils.
EVALUATION OF THE PATIENT WITH GRANULOCYTOSIS
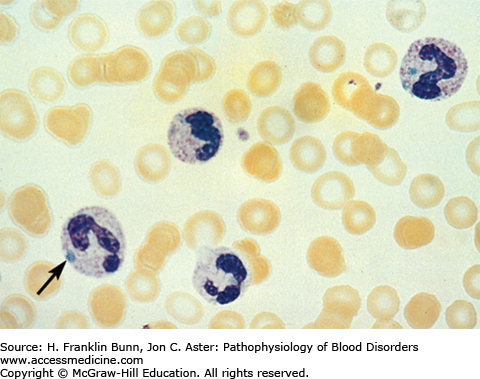
Neutrophilia is often detected during the evaluation of acutely ill patients suspected of having infections. In patients with acute infections, neutrophils often contain toxic granulation (persistence of primary granules), cytoplasmic vacuoles, and Döhle bodies, pale blue cytoplasmic aggregates of endoplasmic reticulum (Fig. 18-3). In severe sepsis, phagocytosed organisms occasionally may be seen within neutrophils. In acutely ill patients, the primary clinical focus is on the identification of infectious agents and the institution of appropriate antibiotic therapy.
FIGURE 18-3
Döhle bodies and toxic granulation, peripheral smear. An arrow denotes a light blue Döhle 1in the cytoplasm of a neutrophil. In addition, several neutrophils contain toxic granulations, coarse purple cytoplasmic granules that are best appreciated in the cell in the upper right part of the field. In many patients with severe infections, neutrophils with both Döhle bodies and toxic granulations may be seen.
The differential diagnosis of neutrophilia in sub-acutely or chronically ill patients is quite broad and includes numerous infectious, inflammatory, and neoplastic disorders. Mild neutrophilia in asymptomatic patients can be related to smoking or subtle inflammatory disease but requires evaluation to rule out important acquired diseases such as chronic myelogenous leukemia.
Neutrophilia is usually reactive and indicative of a normal-functioning bone marrow; consequently, bone marrow evaluation is usually not necessary. The exceptions are neutrophilias associated with either leukoerythroblastosis or the presence of immature myeloid precursors in the peripheral blood. Leukoerythroblastosis is indicative of an infiltrative disorder in the marrow, such as metastatic cancer, primary bone marrow disorders associated with fibrosis, or a disseminated granulomatous disease such as tuberculosis. The presence of immature myeloid precursors (often in concert with eosinophilia and basophilia) is characteristic of myeloid neoplasms such as chronic myelogenous leukemia and polycythemia vera, both of which can be distinguished from reactive processes by molecular tests described in Chapter 20. In unexplained persistent neutrophilia, a bone marrow examination may be informative diagnostically and should always include cultures for tuberculosis and fungal pathogens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree