Leiomyosarcoma
Elizabeth A. Montgomery, MD
Key Facts
Terminology
Malignant neoplasm composed of cells exhibiting smooth muscle differentiation
Etiology/Pathogenesis
Epstein-Barr virus-associated in immunosuppressed patients
Clinical Issues
Retroperitoneal and inferior vena cava lesions more common in women
Deep soft tissue mass, often asymptomatic in extremities
Retroperitoneum most common site
Site and stage dependent as per other sarcoma types
Surgical excision
Rare: 10-15% of extremity sarcomas
Most common sarcoma type if uterine examples are included
Microscopic Pathology
Perpendicularly oriented fascicles of spindle cells
Brightly eosinophilic cytoplasm
Blunt-ended nuclei
Nuclear atypia
Ancillary Tests
Labels as per smooth muscle: Desmin, actin, calponin, caldesmon
Some cases label with keratins
Top Differential Diagnoses
Gastrointestinal stromal tumor (in gastrointestinal tract)
Fibromatosis (in most sites)
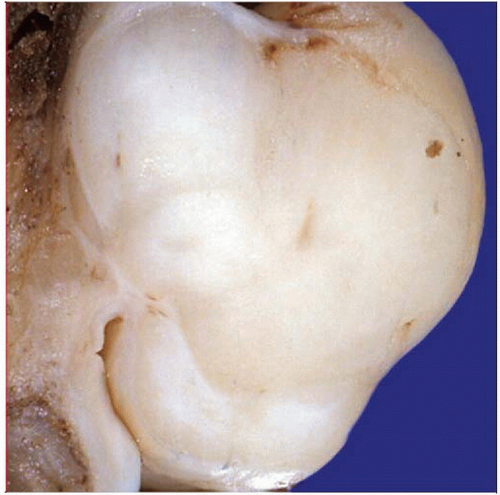 Gross pathology photograph shows a leiomyosarcoma arising in association with a large deep vessel. This is a common presentation of leiomyosarcomas. |
TERMINOLOGY
Abbreviations
Leiomyosarcoma (LMS)
Definitions
Malignant neoplasm composed of cells exhibiting smooth muscle differentiation
ETIOLOGY/PATHOGENESIS
Infectious Agents
Epstein-Barr virus associated in immunosuppressed patients
Occasional examples associated with radiation
CLINICAL ISSUES
Epidemiology
Incidence
Rare: 10-15% of extremity sarcomas
Most common overall sarcoma type if uterine and visceral examples are included
Age
Middle-aged adults
Gender
No gender preference overall
Retroperitoneal and inferior vena cava lesions more common in women
Presentation
Deep soft tissue mass, often asymptomatic in extremities
Retroperitoneum most common site
Retroperitoneal lesions can be associated with abdominal pain
Vena cava examples often symptomatic
Upper portion: Budd-Chiari syndrome (hepatomegaly, jaundice, ascites)
Mid-portion: Renal obstruction
Lower portion: Lower extremity edema
Uterine examples considered separately with unique diagnostic criteria
Treatment
Surgical excision
Radiation
Chemotherapy
Prognosis
Outcome depends on site and stage as per other sarcoma types
Lesions restricted to cutis essentially never metastasize
Some observers have advocated diagnosing them as “atypical smooth muscle tumors”
Subcutaneous lesions
Up to 1/3 of tumors metastasize
10-20% of patients with subcutaneous lesion die of leiomyosarcoma
Retroperitoneum: About 80% of patients die of disease, typically with metastases
Bone: Metastases in up to 1/2 of patients
5-year survival: 65%
Vena cava: 5- and 10-year survival: 50% and 30%, respectively
Head and neck
Few data available
Over 1/2 metastasize
MICROSCOPIC PATHOLOGY
Histologic Features
Perpendicularly oriented fascicles of spindle cells
Brightly eosinophilic cytoplasm
Blunt-ended nuclei
Nuclear atypia
Some examples are epithelioid
Any number of mitoses sufficient in subcutis, scrotal lesions, or deep soft tissue if nuclear atypia is present
In vulva, some observers offered > 5 mitosis per 10 HPF as “cutoff,” but recurrences reported in lesions with any mitotic activity
In uterus
Diffuse moderate to marked cytologic atypia and
Mitotic rate 10 or more mitoses per 10 HPFs and
Tumor cell necrosis
Predominant Pattern/Injury Type
Fascicular
Predominant Cell/Compartment Type
Mesenchymal, muscle, smooth
Variant and Special Forms
Myxoid leiomyosarcoma
Grossly gelatinous
Extensive myxoid change, but zones of typical leiomyosarcoma allow diagnosis
Express desmin and actin
Subset labels with keratin antibodies
Tends to be low grade
Clinicopathologic features otherwise as per typical leiomyosarcoma
Inflammatory leiomyosarcoma
Characterized by dense inflammation that masks underlying lesion
Histiocytes, xanthoma cells, lymphocytes, neutrophils
Areas of more typical morphology must be sought
Clinicopathologic features otherwise as per typical leiomyosarcoma
Pleomorphic leiomyosarcoma
Defined as pleomorphic areas in > 2/3 of tumor
Ordinary leiomyosarcomatous fascicular area covers < 1/3
More aggressive since higher grade
In 1 series, 65% of patients died of disease
Subset features osteoclast-like giant cells
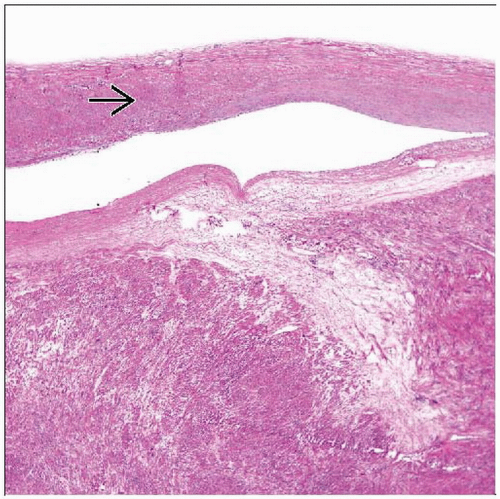
Epstein-Barr-virus-associated leiomyosarcoma
a.k.a. Epstein-Barr-virus-associated smooth muscle tumors (EBV-SMT)
Regarded as “leiomyoma” and “leiomyosarcoma,” but term EBV-SMT may be more appropriate
Appearances are somewhat unique
Found in immunosuppressed patients
Frequently multifocal
Each tumor is unique molecular event; no clearcut metastases reported
Histologic features
Monomorphic, spindled, smooth muscle cells arranged in short intersecting fascicles
Subpopulation of more primitive round cells are either admixed with spindled cells or form discrete nodules
Variable lymphocytic infiltrate composed primarily of T cells
Mitotic activity variable (0-18 per 10 HPF)
Necrosis and myxoid change in some cases
All are EBV-encoded RNA (EBER) positive
All express SMA, desmin in ˜ 1/2
Reducing immunosuppression in transplant patients should be key treatment
Rapid tumor reduction following reduced immunosuppression reported, but some lesions persist
About 5% die of disease
Treatment is primarily surgical
Sirolimus (inhibitor of mTOR-associated protein pathway) effective in some lesions
Leiomyosarcoma with osteoclast-like giant cells
Same demographics as per typical leiomyosarcoma
Areas with same histology as per typical leiomyosarcoma
Reactive with smooth muscle markers: Actins and desmin
Areas with osteoclast-like giant cells
Some giant cells appear bland (like histiocytes), but others cytologically malignant
Benign-appearing osteoclast-like giant cells label with CD68 but not muscle markers
Cytologically malignant giant cells label with smooth muscle markers
No osteoid/matrix formation seen
Epithelioid leiomyosarcoma
Literature confounded because many epithelioid gastrointestinal stromal tumors (GIST) were termed epithelioid leiomyosarcoma in past
Found anywhere in body
Distinct epithelioid morphology but more nuclear atypia than gastrointestinal stromal tumors
Older studies reported actin(+), desmin(-) immunophenotype, but desmin labels most lesions using modern immunohistochemistry
Possible reflection of misdiagnosed GISTs
Less sensitive desmin antibodies in past
ANCILLARY TESTS
Immunohistochemistry
Label as per smooth muscle: Desmin, actin, calponin, caldesmon
Some cases label with keratins
Cytogenetics
Complex variable karyotypes
No characteristic translocation, mutation, or fusion product known
DIFFERENTIAL DIAGNOSIS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree