THERAPEUTIC AND TOXIC CONCENTRATIONS
Unlike most of the older antiepileptic drugs, the therapeutic range of lamotrigine is not well defined.13–16 However, lamotrigine serum concentration measurement can be a useful adjunct in addition to monitoring treatment for therapeutic response and adverse effects. The therapeutic range for epilepsy used by many laboratories for lamotrigine is 2-20 μg/mL. Some patients will experience a higher incidence of adverse effects at concentrations above 10-15 μg/mL, so the efficacy of lower serum concentrations should be assessed before moving to higher concentrations.13–15 The most common concentration-dependent side effects of lamotrigine are diplopia, dizziness, ataxia, blurred vision, unsteadiness, and headache.12
CLINICAL MONITORING PARAMETERS
The goal of therapy with anticonvulsants is to reduce seizure frequency and maximize quality of life with a minimum of adverse drug effects.12 While it is desirable to entirely abolish all seizure episodes, it may not be possible to accomplish this in many patients. The goal of therapy with lamotrigine for patients with bipolar disorder is to decrease the frequency and severity of mood episodes with a minimum of side effects. Patients should be monitored for concentration-related side effects (diplopia, dizziness, ataxia, blurred vision, unsteadiness, headache) as well as gastrointestinal upset (nausea, vomiting).12,17,18 Somnolence, tremor, and rash are also common adverse events.12,17,18 Typically, the rash caused by lamotrigine is classified as erythematous, generalized, and morbilliform.9
Serious, life-threatening rashes have been associated with lamotrigine treatment, and the prescribing information for the drug carries a black box warning for Stevens-Johnson syndrome, toxic epidermal necrolysis, and rash-related death.17,18 These types of rashes usually occur early in therapy, typically in the first few months of use.2,12 The manufacture recommends immediate discontinuation of lamotrigine upon development of a rash, unless the rash is proven to be not related to lamotrigine treatment. Some factors that may increase the risk of developing a rash include concurrent administration of valproate, administration of an initial dose of lamotrigine that exceeds the recommended starting dose, and titration to higher doses that exceed the recommended rate for lamotrigine.17,18
Because epilepsy is an episodic disease state, patients do not experience seizures continuously. Thus, during dosage titration it is difficult to tell if the patient is responding to drug therapy or simply is not experiencing any abnormal central nervous system discharges at that time. Similarly, bipolar disorder also occurs at irregular time intervals. Lamotrigine doses should be carefully titrated to individual patient response and tolerability. Serum concentrations of lamotrigine can be measured in patients as an adjunct to therapy.13–16 Concentration monitoring is particularly useful if a therapeutic concentration has been established for a patient and a new clinical event (drug interaction, new disease state or condition, etc) changes the pharmacokinetics of the drug. Lamotrigine concentrations can also be measured to document patient adherence to drug treatment or when a patient is switched between the immediate-release and extended-release dosage forms to assure therapeutic levels are maintained.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Lamotrigine is eliminated primarily via glucuronidation by the uridine 5′-diphospho-glucuronosyltransferase (UDP-glucuronosyltransferase or UGT) system. UGT1A4 appears to be the principle isoenzyme involved in the reaction.19 After single doses of lamotrigine to normal subjects, 86% of the administered dose was recovered as glucuronidated metabolites (76% 2-N-glucuronide, 10% 5-N-glucuronide) and 10% was recovered as unchanged lamotrigine.20–23 Lamotrigine follows linear pharmacokinetics within the typical dosage range.11,24–26
For oral use, lamotrigine is available in a variety of immediate-release tablets (tablets: 25 mg, 50 mg, 100 mg, 150 mg, 200 mg; chewable tablets: 2 mg, 5 mg, 25 mg; orally disintegrating tablets: 25 mg, 50 mg, 100 mg) and as extended-release tablets (25 mg, 50 mg, 100 mg, 200 mg, 250 mg, 300 mg). Chewable tablets can be swallowed whole, chewed, crushed, or dispersed in water or fruit juice for administration. Disintegrating tablets are placed on the tongue and then they are swished around in the mouth. If desired, water can be used to help swallow the medication after disintegration.
During multiple dosing, peak concentrations occur at about the same time (Tmax) for all of immediate-release dosage forms (1.5-2.0 hours) when given twice daily.25,27,28 For multiple doses of the extended-release dosage form administered once daily, Tmax depends on the other medications that patients take.17,29,30 During concurrent therapy with hepatic enzyme inducers (carbamazepine, phenytoin, phenobarbital, and/or primidone), the Tmax for extended-release lamotrigine tablets is 4-6 hours. When given concomitantly with valproic acid, Tmax for lamotrigine extended-release tablets equals 9-11 hours. When administered with other antiepileptic drugs, lamotrigine extended-release tablets have a Tmax of 6-10 hours. If patients take other antiepileptic drugs that induce hepatic enzymes (such as carbamazepine, phenytoin, phenobarbital, or primidone) and switch from immediate-release lamotrigine to the identical daily dose of extended-release lamotrigine, area under the serum concentration curve may be reduced by clinically significant amounts (as much as 70%). When patients are switched between dosage forms, they should be closely monitored for changes in efficacy or toxicity, and measurement of lamotrigine serum concentrations should be considered before and after the change has been made.
Absorption of orally administered lamotrigine is nearly 100% and is not affected by concomitant administration with food.16,31,32 Specific initial dosage titration is recommended to avoid adverse effects, so the manufacturers of lamotrigine provide special packaging of the medication in the form of starter packs or titration kits as an adherence aid for patients.
The plasma protein binding of lamotrigine is 55%, and the average volume of distribution ranges from 0.9 to 1.3 L/kg.16,25,27,28,32,33 Lamotrigine crosses the placental barrier, and at birth the umbilical cord blood to maternal serum concentration ratio is approximately 1.0.34–36 Lamotrigine also crosses into the breast milk of lactating mothers with a breast milk to maternal serum ratio of 0.59 (range: 0.35-0.86).34,36 After breastfeeding from mothers taking lamotrigine (median maternal lamotrigine serum concentration = 9.0 μg/mL), the resulting median lamotrigine serum concentration in four newborns was 2.2 μg/mL.36 In individual neonates and young infants, serum lamotrigine concentrations have been measured as high as 50% of the concurrent maternal amount in the serum.17 The lamotrigine saliva-to-serum ratio after single doses is 0.46.27
EFFECTS OF DISEASES AND CONDITIONS ON PHARMACOKINETICS AND DOSING
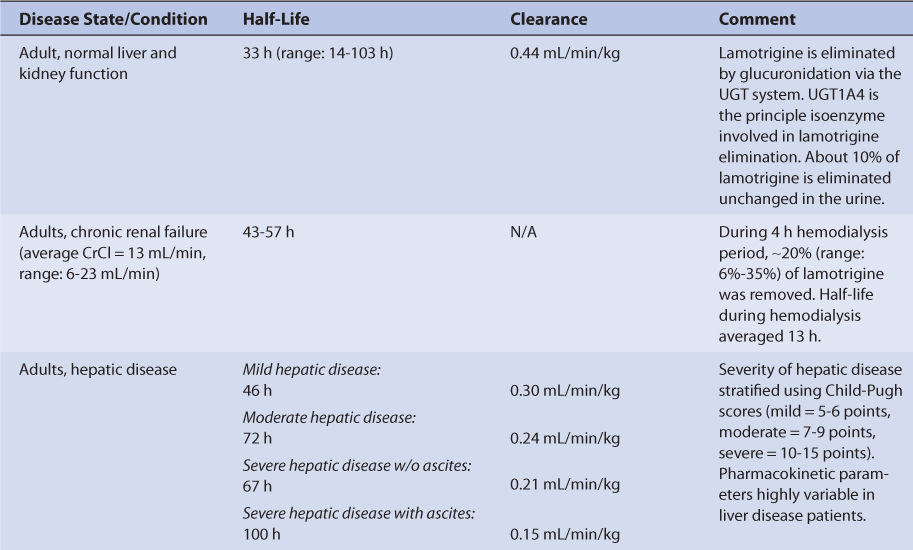
Adults with normal liver and kidney function that take no hepatic enzyme inducers or inhibitors have an average half-life of 33 hours and an average clearance of 0.44 mL/min/kg for lamotrigine (Table 15-2).27,37–40 The volume of distribution of lamotrigine is 0.9-1.3 L/kg. Because plasma protein binding is moderate (~55%), it is not altered to a large degree by the disease states or conditions that alter lamotrigine pharmacokinetics, and volume of distribution is similar for most patients.16,25,27,28,32,33
Since a small amount of lamotrigine is eliminated unchanged in the urine (~10%), there is only a modest increase in average half-life for subjects with renal failure, and the range for these individuals falls within the range of adults with normal kidney function.22,23,26,41 During a 4-hour hemodialysis session, about 20% of the total body amount of lamotrigine is removed, and the lamotrigine half-life during the procedure decreases to a mean value of 13 hours.42,43 For hemodialysis patients receiving lamotrigine treatment, serum concentration measurement before and after the dialysis procedure can be helpful to ascertain the need for a posthemodialysis replacement dose.
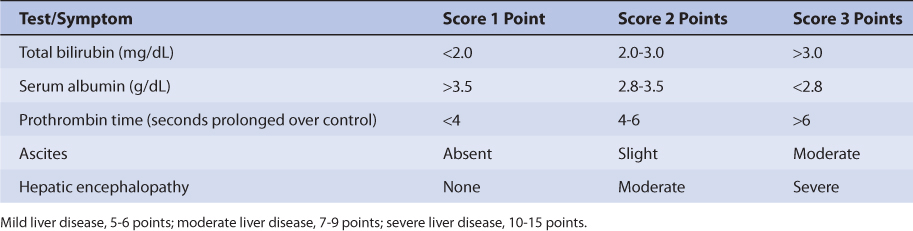
The majority of a lamotrigine dose (~90%) is eliminated by glucuronidation via UGT, and UGT1A4 appears to be the principal isozyme involved.19–23 Because of this, hepatic disease prolongs lamotrigine half-life and decreases lamotrigine clearance in a graded manner according to the degree of liver damage observed in the patient.44 An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 15-3).45 Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; see Table 15-2), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. The pharmacokinetic changes for lamotrigine range from modest ones (half-life = 46 hours, clearance = 0.30 mL/min/kg) for patients with mild liver disease (Child-Pugh score = 5-6 points) to large ones (half-life = 100 hours, clearance = 0.15 mL/min/kg) for patients with severe liver disease that includes ascites (Child-Pugh score = 10-15 points, that includes ascites as one of the scored characteristics).17,18,44 Generally, no change in dose is needed for patients with mild liver disease. But, for patients with moderate or severe liver disease, decreases are needed for initial, escalation, and maintenance doses (~25% for moderate or severe liver disease without ascites, ~50% for severe liver disease with ascites). Escalation and maintenance doses should be titrated to clinical response, and measurement of lamotrigine serum concentrations can be a helpful adjunct. Because there is a large amount of variability in pharmacokinetics, patients with liver disease should be closely monitored for adverse effects due to lamotrigine therapy.
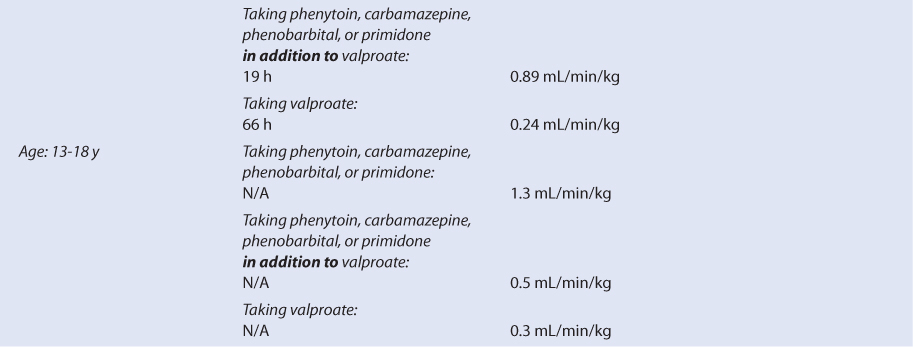
Pediatric patients taking lamotrigine for the treatment of seizures have been studied while taking other antiepileptics medications, so it is important to take concurrent medications into account when interpreting the findings of pharmacokinetic studies (see Table 15-2).46–58 For children between the ages of 10 months and 5.3 years taking other antiepileptic drugs that are not thought to cause drug interactions with lamotrigine, the mean lamotrigine half-life was 19 hours and the mean lamotrigine clearance was 1.2 mL/min/kg. This pattern of higher clearance and shorter half-life compared to adults is observed with most other medications. The expected pattern of pharmacokinetic changes is also found in pediatric patients taking other antiepileptic drugs that are hepatic enzyme inducers (phenytoin, carbamazepine, phenobarbital, primidone: lamotrigine clearance = 3.6 mL/min/kg, lamotrigine half-life = 8 hours) or hepatic enzyme inhibitors (valproate: lamotrigine clearance = 0.47 mL/min/kg, lamotrigine half-life = 45 hours). Similar situations are observed with lamotrigine pharmacokinetics for older children aged 5-11 years and 13-18 years. When these age groups are administered lamotrigine concurrently with antiepileptics that are hepatic enzyme inducers, lamotrigine clearance is higher and lamotrigine half-life is shorter compared to adults, but there is a gradual change toward adult values as age increases, especially in teenaged patients. For the two older pediatric patient groups, it is also possible to see the effects of mixed coadministration of antiepileptic drugs that are hepatic enzyme inducers and inhibitors. In these cases, concomitant administration of valproate modulates the induction effect of phenytoin, carbamazepine, phenobarbital, or primidone therapy on lamotrigine pharmacokinetics.
Lamotrigine disposition has also been investigated in older adults (age range: 65-76 years, mean CrCl: 61 mL/min).38 In this investigation, the pharmacokinetic parameters for lamotrigine was very similar to those measured in younger adults (see Table 15-2). Note that the subjects in this study had relatively good renal function for their age. Many elderly patients will have poorer renal function, and these individuals may have some moderate changes in the pharmacokinetics of lamotrigine.59
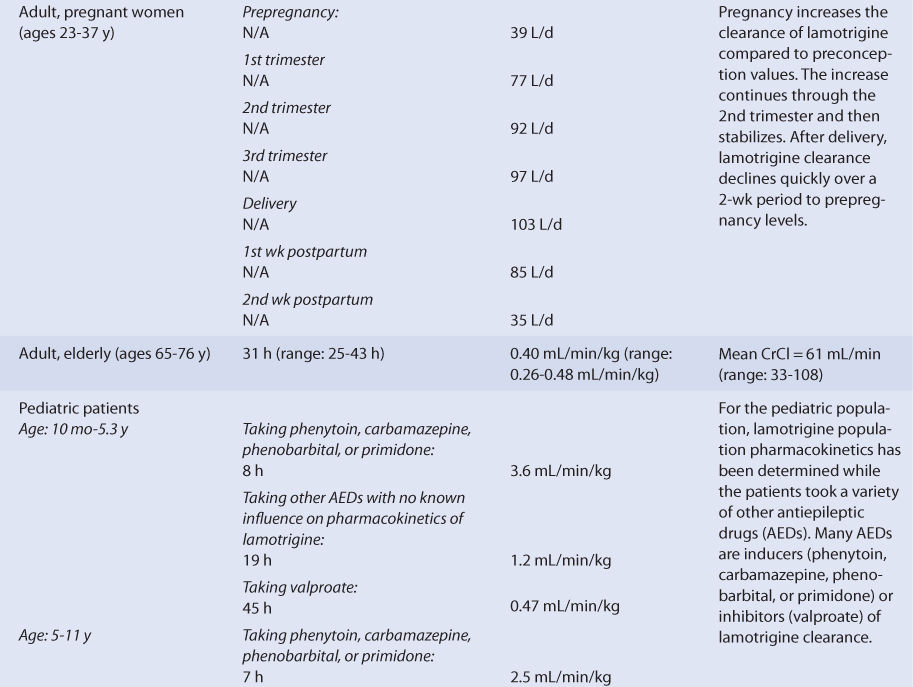
Using the FDA pregnancy classification system, lamotrigine is a Category C medication. So, lamotrigine is used to treat pregnant women when the potential benefits of drug therapy outweigh the potential risks. The clearance of lamotrigine is altered as the pregnancy progresses (see Table 15-2).36 Compared to prepregnancy values, clearance increases through the first trimester and finally stabilizes during the second semester. Clearance remains increased until delivery, then starts to decline. By the second postpartum week, lamotrigine clearance returns to baseline values.
DRUG INTERACTIONS
Lamotrigine is eliminated via glucuronidation by the UGT system.20–23 UGT1A4 is thought to be the principal isozyme involved in this reaction.19 Lamotrigine pharmacokinetics are affected by enzyme inducers and inhibitors of this enzymatic pathway.60,61 The principal enzyme inducers of lamotrigine clearance are the other antiepileptic drugs phenytoin, carbamazepine, phenobarbital, or primidone, and lamotrigine is commonly used in combination with these agents.25,28,51,52,56,62 Valproate is the principal enzyme inhibitor of lamotrigine clearance.28,40,51,52,56,62 Specific initial, escalation, and maintenance doses are recommended for patients taking combinations of one or more of these inducers and/or this inhibitor of lamotrigine clearance (Tables 15-4 to 15-6).17,18 Obviously, these drug interactions may take place whether the patient is being treated for seizures or bipolar disorders.
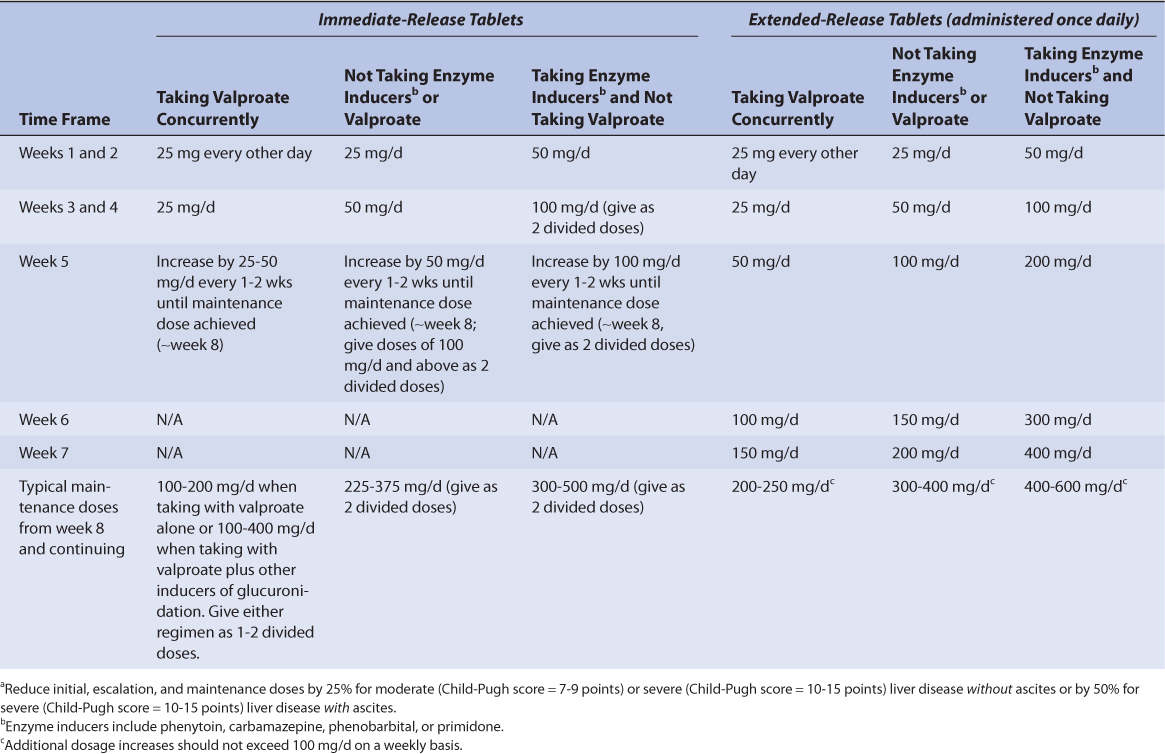
TABLE 15-4 Initial, Escalation, and Maintenance Doses of Lamotrigine for Initial Adjunctive Therapy of Epilepsy for Patients 13 Years or Oldera
Studies conducted in patients or subjects taking single or multiple doses of lamotrigine indicate that lamotrigine clearance is increased by 109%-150% when taken with phenytoin, carbamazepine, phenobarbital, or primidone (adults: mean Cl = 1.1-1.2 mL/min/kg, mean t1/2 = 12-14 hours; children: see Table 15-2). However, when one of these other antiepileptic drugs is taken with valproate, lamotrigine clearance is decreased 20% from baseline (adults: mean Cl = 0.53 mL/min/kg, mean t1/2 = 27 hours; children: see Table 15-2). When lamotrigine is taken only with valproate, lamotrigine clearance decreases by 32%-69% (adults: mean Cl = 0.28 mL/min/kg, mean t1/2 = 59 hours; children: see Table 15-2). Clearly, valproate is such a potent inhibitor of lamotrigine elimination that it is able to negate the induction of metabolism due to several other antiepileptic drugs.
Other hepatic enzyme inducers of lamotrigine include efavirenz, nevirapine, oxcarbazepine, rifabutin, rifampin, rifapentine, and St. John’s wort.60 Oral contraceptives that contain an estrogen component (most commonly ethinyl estradiol) also induce lamotrigine clearance.60,61 When taken concurrently with an ethinyl estradiol–containing oral contraceptive, lamotrigine clearance nearly doubled. However, during the placebo or drug-free week of oral contraceptive therapy in women taking the medication cyclically in order to induce withdrawal bleeding, lamotrigine concentrations increased twofold in 7 days. One recommended solution to this situation is to use an oral contraceptive with a different hormone composition.60 Specific recommendations for maintenance doses of lamotrigine are available for patients starting, stopping, or taking estrogen-containing oral contraceptives (see Initial Dosage Determination Methods section).
Lamotrigine therapy may increase the carbamazepine-10,11-epoxide to carbamazepine ratio in some patients.63,64 Because the epoxide is an active metabolite with pharmacology similar to carbamazepine, patients taking lamotrigine and carbamazepine together may experience enhanced therapeutic effects or toxicities of carbamazepine with a decrease or no change in carbamazepine serum concentrations.61
INITIAL DOSAGE DETERMINATION METHODS
Although the risk factors for developing life-threatening rashes due to lamotrigine are not completely understood, there are suggestions in the literature that the incidence may be increased by concomitant administration of valproate, administration of initial lamotrigine doses that exceed the maximum recommended amount, or escalation of lamotrigine doses during the titration phase that exceeds the maximum recommended rate. Because of this, initial doses of lamotrigine are low, and the rate of dosage increase to target therapeutic amounts is slow. Additionally, initial dose and rate of dosage increase are adjusted to reflect changes in lamotrigine pharmacokinetics due to other drug therapy and disease states or conditions that the patient may have. While some clinicians prefer to use custom algorithms for initial doses and dosage titration of lamotrigine, many use the literature-based recommendations given in the prescribing information for lamotrigine because of the potential risk and ramifications of developing a rash during therapy (Tables 15-4 to 15-7).17,18
When used as adjunctive therapy for epilepsy, initial, escalation, and target doses for lamotrigine are stratified by age, dosage form, and concurrent drug therapy (see Tables 15-4 and 15-5). For pediatric patients weighing <30 kg, maintenance doses may need to be up to 50% higher for all ages and concurrent drug therapy categories (see Table 15-5). For initiation and escalation of lamotrigine as monotherapy, the recommendations for “not taking enzyme inducers or valproate” can be used.
TABLE 15-5 Escalation Doses of Lamotrigine for Initial Adjunctive Therapy of Epilepsy for Patients 2-12 Yearsa

Each category contains escalation dosage titrations that take several weeks to attain the target dosage. Because the titration process is complicated, some manufacturers have made available titration blister packs for the various dosage scenarios that may aid patient adherence to the regimen. Similarly, initial, escalation, and target doses of lamotrigine for bipolar disorders are stratified by concurrent drug therapy (see Table 15-6).
TABLE 15-6 Escalation Doses of Immediate-Release Lamotrigine for Patients With Bipolar Disorder for Patients 18 Years and Older
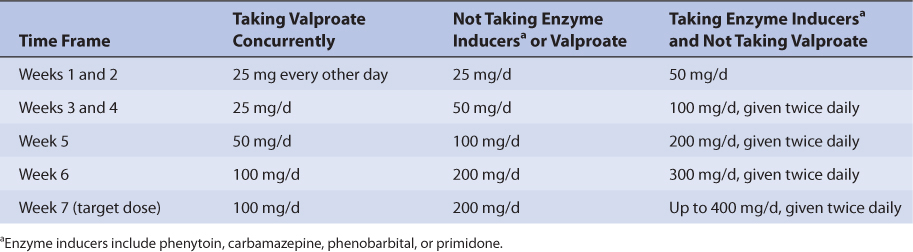
Specific recommendations are available to convert epileptic patient drug therapy from concurrent therapy with lamotrigine and valproate to monotherapy with lamotrigine (see Table 15-7). Conversion of concurrent drug therapy with other antiepileptic drugs that are hepatic enzyme inducers (phenytoin, carbamazepine, phenobarbital, primidone) and lamotrigine to monotherapy with lamotrigine is conducted in two steps. First, using the recommendations in Table 15-4 for patients taking enzyme inducers with lamotrigine, establish a lamotrigine dose of 500 mg/d. Second, withdraw the other antiepileptic drug by reducing the dose by 20%, then continuing the same weekly decrease for 4 weeks until it is discontinued. For the extended-release tablets only, the target dose for lamotrigine monotherapy is 250-300 mg/d (compared to 500 mg/d for immediate-release lamotrigine tablets) so additional lamotrigine dosage titration is needed for this dosage form. After an additional 2 weeks have passed to allow hepatic enzyme induction to diminish, the lamotrigine extended-release dose may be decreased by 50-100 mg/d on a weekly basis, as needed, until the target dose is attained.
TABLE 15-7 Conversion to Monotherapy With Immediate-Release or Extended-Release Lamotrigine From Adjunctive Treatment With Concurrent Valproate for the Treatment of Epilepsy
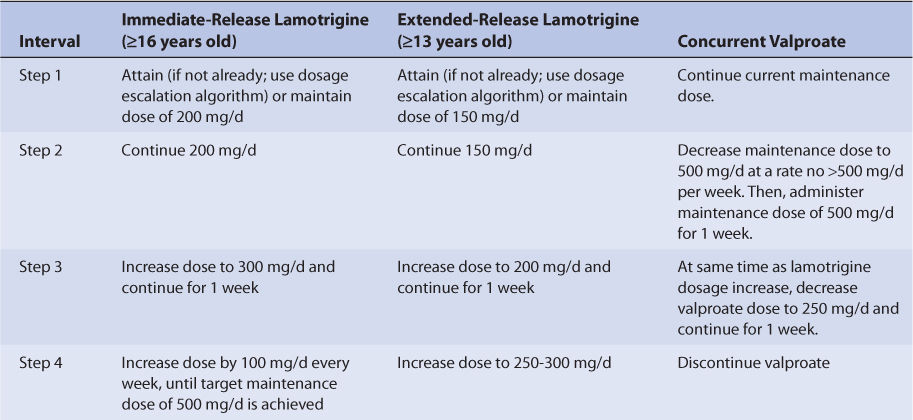
Although estrogen-containing oral contraceptives (principally those containing ethinyl estradiol, not progestin-only pills) are known to increase the clearance of lamotrigine, the recommended initial, escalation, and titration schedules for initiation of lamotrigine treatment should be followed in women taking these types of birth control pills (see Table 15-4). Eventually, maintenance doses for these individuals may need to be increased by as much as twofold according to clinical response. For women already receiving a stable maintenance dose of lamotrigine, starting estrogen-containing oral contraceptive therapy will probably require an increased maintenance dose of lamotrigine. When this is the case, lamotrigine dosage increases can be started at the same time as the estrogen-containing oral contraceptive, and the rate of dosage increase should be no greater than 50-100 mg/d on a weekly basis. Additionally, dose increases should not be greater than recommended by the disease state–specific escalation recommendations (see Tables 15-4 and 15-6) unless needed to maintain clinical response or effective lamotrigine serum concentrations. If the patient is taking cyclic estrogen-containing oral contraceptives with placebo or no tablets for a week, lamotrigine serum concentrations can increase during this time period, causing adverse effects. In this case, doses of lamotrigine may need to be decreased for the entire 28-day cycle. For patients already taking other inducers of glucuronidation (phenytoin, carbamazepine, phenobarbital, primidone, rifampin, etc), it may not be necessary to change maintenance doses of lamotrigine when oral contraceptive therapy is started.
When women stop taking estrogen-containing oral contraceptives, lamotrigine maintenance doses may need to be decreased by as much as 50%.17,18 Doses should be decreased slowly in a stepwise fashion, no faster than 25% decrements per week over a 2-week time frame unless clinical response or lamotrigine serum concentrations support a different approach. For women taking other inducers of glucuronidation (phenytoin, carbamazepine, phenobarbital, primidone, rifampin, etc), it may not be necessary to alter maintenance doses of lamotrigine.
Generally, no change in dose is needed for patients with mild liver disease (Child-Pugh score = 5-6 points). But, for patients with moderate (Child-Pugh score = 7-9 points) or severe liver disease (Child-Pugh score = 10-15 points), decreases are needed for initial, escalation, and maintenance doses (~25% for moderate or severe liver disease without ascites, ~50% for severe liver disease with ascites). Escalation and maintenance doses should be titrated to clinical response, and measurement of lamotrigine serum concentrations can be a helpful adjunct. Of course, patients with liver disease should be closely monitored for adverse effects due to lamotrigine therapy.
Since only a small amount of lamotrigine is eliminated unchanged in the urine (~10%), dosing changes for patients with renal failure are usually not necessary. However, hemodialysis does clear lamotrigine from the body, and during a 4-hour hemodialysis session, about 20% of the total body amount of lamotrigine is removed. For hemodialysis patients receiving lamotrigine treatment, serum concentration measurement before and after the dialysis procedure can be helpful to ascertain the need for a posthemodialysis replacement dose.
If discontinuation of lamotrigine is necessary due to lack of therapeutic response or to development of adverse effects, the dosage should be tapered for patients taking the medication for either epilepsy or bipolar disorder. Unless the clinical situation dictates otherwise, lamotrigine doses should be decreased by about 50% per week over at least 2 weeks.
Literature-Based Recommended Dosing
The following examples illustrate the use of literature-based recommended dosing for different patient situations.