Key Terms
Airborne pathogens
Biohazard
Bloodborne pathogens
Carcinogens
Chemical hygiene plan
Corrosive chemicals
Cryogenic material
Fire tetrahedron
Hazard Communication Standard
Hazardous materials
High-efficiency particulate air (HEPA) filters
Laboratory standard
Mechanical hazards
Medical waste
National Fire Protection Association (NFPA)
Occupational Safety and Health Act (OSHA)
Radioactive materials
Reactive chemicals
Safety Data Sheets (SDSs)
Teratogens
Universal precautions
LABORATORY SAFETY AND REGULATIONS
Clinical laboratory personnel, by the nature of the work they perform, are exposed daily to a variety of real or potential hazards: electric shock, toxic vapors, compressed gases, flammable liquids, radioactive material, corrosive substances, mechanical trauma, poisons, and the inherent risks of handling biologic materials, to name a few. Each clinician should develop an understanding of the risks associated with these hazards and must be “safety conscious” at all times.
Laboratory safety necessitates the effective control of all hazards that exist in the clinical laboratory at any given time. Safety begins with the recognition of hazards and is achieved through the application of common sense, a safety-focused attitude, good personal behavior, good housekeeping in all laboratory work and storage areas, and, above all, the continual practice of good laboratory technique. In most cases, accidents can be traced directly to two primary causes: unsafe acts (not always recognized by personnel) and unsafe environmental conditions. This chapter discusses laboratory safety as it applies to the clinical laboratory.
Occupational Safety and Health Act
Public Law 91-596, better known as the Occupational Safety and Health Act (OSHA), was enacted by the U.S. Congress in 1970. The goal of this federal regulation was to provide all employees (clinical laboratory personnel included) with a safe work environment. Under this legislation, the Occupational Safety and Health Administration (also known as OSHA) is authorized to conduct on-site inspections to determine whether an employer is complying with the mandatory standards. Safety is no longer only a moral obligation but also a federal law. In about half of the states, this law is administered by individual state agencies rather than by the federal OSHA. These states still fall within delineated OSHA regions, but otherwise they bear all administrative, consultation, and enforcement responsibilities. The state regulations must be at least as stringent as the federal ones, and many states incorporate large sections of the federal regulations verbatim.
OSHA standards that regulate safety in the laboratory include the Bloodborne Pathogen Standard, Formaldehyde Standard, Laboratory Standard, Hazard Communication Standard, Respiratory Protection Standard, Air Contaminants Standard, and Personal Protective Equipment Standard. Because laws, codes, and ordinances are updated frequently, current reference materials should be reviewed. Assistance can be obtained from local libraries, the Internet, and federal, state, and local regulatory agencies. The primary standards applicable to clinical laboratory safety are summarized next.
Bloodborne Pathogens [29 CFR 1910.1030]
This standard applies to all exposure to blood or other potentially infectious materials in any occupational setting. It defines terminology relevant to such exposures and mandates the development of an exposure control plan. This plan must cover specific preventative measures including exposure evaluation, engineering controls, work practice controls, and administrative oversight of the program. Universal precautions and personal protective equipment (PPE) are foremost among these infection control measures. The universal precautions concept is basically an approach to infection control in which all human blood, tissue, and most fluids are handled as if known to be infectious for the human immunodeficiency virus (HIV), hepatitis B virus (HBV), and other bloodborne pathogens. The standard also provides fairly detailed directions for decontamination and the safe handling of potentially infectious laboratory supplies and equipment, including practices for managing laundry and infectious wastes. Employee information and training are covered regarding recognition of hazards and risk of infection. There is also a requirement for HBV vaccination or formal declination within 10 days of assuming duties that present exposure. In the event of an actual exposure, the standard outlines the procedure for postexposure medical evaluation, counseling, and recommended testing or postexposure prophylaxis.
Hazard Communication [29 CFR 1910.1200]
This subpart to OSHA’s Toxic and Hazardous Substances regulations is intended to ensure that the hazards of all chemicals used in the workplace have been evaluated and that this hazard information is successfully transmitted to employers and their employees who use the substances. Informally referred to as the OSHA “HazCom Standard,” it defines hazardous substances and provides guidance for evaluating and communicating identified hazards. The primary means of communication are through proper labeling, the development and use of safety data sheets (SDSs), and employee education.
Occupational Exposure to Hazardous Chemicals in Laboratories [29 CFR 1910.1450]
This second subpart to OSHA’s Toxic and Hazardous Substances regulations is also known as the “OSHA Lab Standard.” It was intended to address the shortcomings of the Hazard Communication Standard regarding its application peculiar to the handling of hazardous chemicals in laboratories, whose multiple small-scale manipulations differ from the industrial volumes and processes targeted by the original HazCom Standard. The Lab Standard requires the appointment of a chemical hygiene officer and the development of a chemical hygiene plan to reduce or eliminate occupational exposure to hazardous chemicals. This plan is required to describe the laboratory’s methods of identifying and controlling physical and health hazards presented by chemical manipulations, containment, and storage. The chemical hygiene plan must detail engineering controls, PPE, safe work practices, and administrative controls, including provisions for medical surveillance and consultation, when necessary.
Other Regulations and Guidelines
There are other federal regulations relating to laboratory safety, such as the Clean Water Act, the Resource Conservation and Recovery Act (RCRA), and the Toxic Substances Control Act. In addition, clinical laboratories are required to comply with applicable local and state laws, such as fire and building codes. The Clinical and Laboratory Standards Institute (CLSI, formerly National Committee for Clinical Laboratory Standards [NCCLS]) provides excellent general laboratory safety and infection control guidelines in their documents GP17-A3 (Clinical Laboratory Safety; Approved Guideline, Second Edition) and M29-A4 (Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline, Third Edition).
Safety is also an important part of the requirements for initial and continued accreditation of health care institutions and laboratories by voluntary accrediting bodies such as The Joint Commission (TJC; formerly the Joint Commission on Accreditation of Health Care Organizations [JCAHO]) and the Commission on Laboratory Accreditation of the College of American Pathologists (CAP). TJC publishes a yearly accreditation manual for hospitals and the Accreditation Manual for Pathology and Clinical Laboratory Services, which includes a detailed section on safety requirements. CAP publishes an extensive inspection checklist (Laboratory General Checklist) as part of their Laboratory Accreditation Program, which includes a section dedicated to laboratory safety.
Over the past decade, several new laws and directives have been emplaced regarding enhanced security measures for particular hazardous substances with potential for nefarious use in terrorist activities. These initiatives are typically promulgated by the Department of Homeland Security in cooperation with the respective agency regulating chemical, nuclear, or biological agents of concern. Although most laboratories do not store or use the large volumes of chemicals required to trigger chemical security requirements, many laboratories do surpass the thresholds for radiological and biological agents. Management and employees must be cognizant of security requirements for substances in quantities qualifying them for regulation under enhanced security measures for chemical (Chemical Facilities Anti-Terrorism Standards, 6 CFR 27), radiological (Nuclear Regulatory Commission [NRC] Security Orders and Increased Controls for licensees holding sources above Quantities of Concern), and biological (Select Agents and Toxins, 42 CFR 73) agents. Most security measures involve restriction of access to only approved or authorized individuals, assessment of security vulnerabilities, secure physical containment of the agents, and inventory monitoring and tracking.
SAFETY AWARENESS FOR CLINICAL LABORATORY PERSONNEL
Safety Responsibility
The employer and the employee share safety responsibility. While the individual employee has an obligation to follow safe work practices and be attentive to potential hazards, the employer has the ultimate responsibility for safety and delegates authority for safe operations to laboratory managers and supervisors. In order to ensure clarity and consistency, safety management in the laboratory should start with a written safety policy. Laboratory supervisors, who reflect the attitudes of management toward safety, are essential members of the safety program.
Employer’s Responsibilities
- Establish laboratory work methods and safety policies.
- Provide supervision and guidance to employees.
- Provide safety information, training, PPE, and medical surveillance to employees.
- Provide and maintain equipment and laboratory facilities that are free of recognized hazards and adequate for the tasks required.
The employee also has a responsibility for his or her own safety and the safety of coworkers. Employee conduct in the laboratory is a vital factor in the achievement of a workplace without accidents or injuries.
Employee’s Responsibilities
- Know and comply with the established laboratory safe work practices.
- Have a positive attitude toward supervisors, coworkers, facilities, and safety training.
- Be alert and give prompt notification of unsafe conditions or practices to the immediate supervisor and ensure that unsafe conditions and practices are corrected.
- Engage in the conduct of safe work practices and use of PPE.
Signage and Labeling
Appropriate signs to identify hazards are critical, not only to alert laboratory personnel to potential hazards but also to identify specific hazards that may arise because of emergencies such as fire or explosion. The National Fire Protection Association (NFPA) developed a standard hazard identification system (diamond-shaped, color-coded symbol), which has been adopted by many clinical laboratories. At a glance, emergency personnel can assess health hazards (blue quadrant), flammable hazards (red quadrant), reactivity/stability hazards (yellow quadrant), and other special information (white quadrant). In addition, each quadrant shows the magnitude of severity, graded from a low of 0 to a high of 4, of the hazards within the posted area. (Note the NFPA hazard code symbol in Fig. 2.1.)

FIGURE 2.1 Sample chemical label: (1) statement of hazard, (2) hazard class, (3) safety precautions, (4) NFPA hazard code, (5) fire extinguisher type, (6) safety instructions, (7) formula weight, and (8) lot number. Color of the diamond in the NFPA label indicates hazard: Red = flammable. Store in an area segregated for flammable reagents. Blue = health hazard. Toxic if inhaled, ingested, or absorbed through the skin. Store in a secure area. Yellow = reactive and oxidizing reagents. May react violently with air, water, or other substances. Store away from flammable and combustible materials. White = corrosive. May harm skin, eyes, or mucous membranes. Store away from red-, blue-, and yellow-coded reagents. Gray = presents no more than moderate hazard in any of the categories. For general chemical storage. Exception = reagent incompatible with other reagents of same color bar. Store separately. Hazard code (4)—Following the NFPA use, each diamond shows a red segment (flammability), a blue segment (health; i.e., toxicity), and a yellow segment (reactivity). Printed over each color-coded segment is a black number showing the degree of hazard involved. The fourth segment, as stipulated by the NFPA, is left blank. It is reserved for special warnings, such as radioactivity. The numeric ratings indicate degree of hazard: 4 = extreme, 3 = severe, 2 = moderate, 1 = slight, and 0 = none according to present data.
(Courtesy of Baxter International Inc.)
Manufacturers of laboratory chemicals also provide precautionary labeling information for users. Information indicated on the product label includes statement of the hazard, precautionary measures, specific hazard class, first aid instructions for internal/external contact, the storage code, the safety code, and personal protective gear and equipment needed. This information is in addition to specifications on the actual lot analysis of the chemical constituents and other product notes (Fig. 2.1). Over the last two decades, there has been an effort to standardize hazard terminology and classification under an internationally recognized guideline, titled the Globally Harmonized System of Classification and Labeling of Hazardous Chemicals (GHS). This system incorporates universal definitions and symbols to clearly communicate specific hazards in a single concise label format (Fig. 2.2). Although not yet law, or codified as a regulatory standard, OSHA is presently working to align the existing Hazard Communication Standard with provisions of the GHS and encourages employers to begin adopting the program.

FIGURE 2.2 Example of a GHS inner container label (e.g., bottle inside a shipping box).
All in-house prepared reagents and solutions should be labeled in a standard manner and include the chemical identity, concentration, hazard warning, special handling, storage conditions, date prepared, expiration date (if applicable), and preparer’s initials.
SAFETY EQUIPMENT
Safety equipment has been developed specifically for use in the clinical laboratory. The employer is required by law to have designated safety equipment available, but it is also the responsibility of the employee to comply with all safety rules and to use safety equipment.
All laboratories are required to have safety showers, eyewash stations, and fire extinguishers and to periodically test and inspect the equipment for proper operation. It is recommended that safety showers deliver 30 to 50 gallons of water per minute at 20 to 50 pounds per square in. (psi) and be located in areas where corrosive liquids are stored or used. Eyewash stations must be accessible (i.e., within 100 ft or 10 s travel) in laboratory areas presenting chemical or biological exposure hazards. Other items that must be available for personnel include fire blankets, spill kits, and first aid supplies.
Mechanical pipetting devices must be used for manipulating all types of liquids in the laboratory, including water. Mouth pipetting is strictly prohibited.
Chemical Fume Hoods and Biosafety Cabinets
Fume Hoods
Fume hoods are required to contain and expel noxious and hazardous fumes from chemical reagents. Fume hoods should be visually inspected for blockages. A piece of tissue paper placed at the hood opening will indicate airflow direction. The hood should never be operated with the sash fully opened, and a maximum operating sash height should be established and conspicuously marked. Containers and equipment positioned within hoods should not block airflow. Periodically, ventilation should be evaluated by measuring the face velocity with a calibrated velocity meter. The velocity at the face of the hood (with the sash in normal operating position) should be 100 to 120 ft per minute and fairly uniform across the entire opening. Smoke testing is also recommended to locate no flow or turbulent areas in the working space. As an added precaution, personal air monitoring should be conducted in accordance with the chemical hygiene plan of the facility.
Biosafety Cabinets
Biological safety cabinets (BSCs) remove particles that may be harmful to the employee who is working with potentially infectious biologic specimens. The Centers for Disease Control and Prevention (CDC) and the National Institutes of Health have described four levels of biosafety, which consist of combinations of laboratory practices and techniques, safety equipment, and laboratory facilities. The biosafety level of a laboratory is based on the operations performed, the routes of transmission of the infectious agents, and the laboratory function or activity. Accordingly, biosafety cabinets are designed to offer various levels of protection, depending on the biosafety level of the specific laboratory (Table 2.1). BSCs should be periodically recertified to ensure continued optimal performance as filter occlusion or rupture can compromise their effectiveness.
TABLE 2.1 Comparison of Biosafety Cabinet Characteristics

BSC, biological safety cabinet; HEPA, high-efficiency particulate air; lfm, linear feet per minute. Adapted from Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories. 5th ed. Washington, DC: U.S. Government Printing Office; 2009.
Chemical Storage Equipment
Safety equipment is available for the storage and handling of hazardous chemicals and compressed gases. Safety carriers should always be used to transport glass bottles of acids, alkalis, or organic solvents in volumes larger than 500 mL, and approved safety cans should be used for storing, dispensing, or disposing of flammables in volumes greater than 1 quart. Steel safety cabinets with self-closing doors are required for the storage of flammable liquids, and only specially designed, explosion-proof refrigerators may be used to store flammable materials. Only the amount of chemical needed for that day should be available at the bench. Gas cylinder supports or clamps must be used at all times, and larger cylinders should be transported with valve caps on, using handcarts.
PPE and Hygiene
The parts of the body most frequently subject to injury in the clinical laboratory are the eyes, skin, and respiratory and digestive tracts. Hence, the use of PPE and proper hygiene is very important. Safety glasses, goggles, visors, or work shields protect the eyes and face from splashes and impact. Contact lenses do not offer eye protection; it is strongly recommended that they not be worn in the clinical chemistry laboratory, unless additional protective eyewear is also utilized. If any solution is accidentally splashed into the eye(s), thorough irrigation is required.
Gloves and rubberized sleeves protect the hands and arms when using caustic chemicals. Gloves are required for routine laboratory use; however, polyvinyl or other nonlatex gloves are an acceptable alternative for people with latex allergies. Certain glove materials offer better protection against particular reagent formulations. Nitrile gloves, for example, offer a wider range of compatibility with organic solvents than do latex gloves. Laboratory coats, preferably with knit-cuffed sleeves, should be full length and buttoned and made of liquid-resistant material. When performing manipulations prone to splash hazards, the laboratory coat should be supplemented with an impermeable apron and/or sleeve garters, constructed of suitable material to guard against the substances. Proper footwear is required; shoes constructed of porous materials, open-toed shoes, and sandals are considered ineffective against spilled hazardous liquids.
Respirators may be required for various procedures in the clinical laboratory. Whether used for biologic or chemical hazards, the correct type of respirator must be used for the specific hazard. Respirators with high-efficiency particulate air (HEPA) filters must be worn when engineering controls are not feasible, such as when working directly with patients with tuberculosis (TB) or when performing procedures that may aerosolize specimens of patients with a suspected or confirmed case of TB. Training, maintenance, and written protocol for use of respirators are required according to the respiratory protection standard.
Each employer must provide (at no charge) laboratory coats, gloves, or other protective equipment to all employees who may be exposed to biologic or chemical hazards. It is the employer’s responsibility to clean and maintain any PPE used by more than one person. All contaminated PPE must be removed and properly cleaned or disposed off before leaving the laboratory.
Hand washing is a crucial component of both infection control and chemical hygiene. After removing gloves, hands should be washed thoroughly with soap and warm water, even if glove breakthrough or contamination is not suspected. The use of antimicrobial soap is not as important as the physical action of washing the hands with water and any mild soap. After any work with highly toxic or carcinogenic chemicals, the face should also be washed.
BIOLOGIC SAFETY
General Considerations
All blood samples and other body fluids should be collected, transported, handled, and processed using universal precautions (i.e., presumed to be infectious). Gloves, gowns, and face protection must be used during manipulations or transfers when splashing or splattering is most likely to occur. Consistent and thorough hand washing is an essential component of infection control. Antiseptic gels and foams may be used at waterless stations between washes, but they should not take the place of an actual hand wash.
Centrifugation of biologic specimens produces finely dispersed aerosols that are a high-risk source of infection. Ideally, specimens should remain capped during centrifugation, or several minutes should be allowed to elapse after centrifugation is complete before opening the lid. As a preferred option, the use of a sealed-cup centrifuge is recommended. These sealed vessels can then be brought to a biosafety cabinet to be opened.
Spills
Any blood, body fluid, or other potentially infectious material spill must be promptly cleaned up, and the area or equipment must be disinfected immediately. Safe cleanup includes the following recommendations:
- Alert others in area of the spill.
- Wear appropriate protective equipment.
- Use mechanical devices to pick up broken glass or other sharp objects.
- Absorb the spill with paper towels, gauze pads, or tissue.
- Clean the spill site using a common aqueous detergent.
- Disinfect the spill site using approved disinfectant or 10% bleach, using appropriate contact time.
- Rinse the spill site with water.
- Dispose off all materials in appropriate biohazard containers.
Bloodborne Pathogens
In December 1991, OSHA issued the final rule for occupational exposure to bloodborne pathogens. To minimize employee exposure, each employer must have a written exposure control plan. The plan must be available to all employees whose duties may result in reasonably anticipated occupational exposure to blood or other potentially infectious materials. The exposure control plan must be discussed with all employees and be available to them while they are working. The employee must be provided with adequate training in all techniques described in the exposure control plan at initial work assignment and annually thereafter. All necessary safety equipment and supplies must be readily available and inspected on a regular basis.
Clinical laboratory personnel are knowingly or unknowingly in frequent contact with potentially biohazardous materials. In recent years, new and serious occupational hazards to personnel have arisen, and this problem has been complicated because of the general lack of understanding of the epidemiology, mechanisms of transmission of the disease, or inactivation of the causative agent. Special precautions must be taken when handling all specimens because of the continual increase in the proportion of infectious samples received in the laboratory. Therefore, in practice, specimens from patients with confirmed or suspected hepatitis, acquired immunodeficiency syndrome (AIDS), or other potentially infectious diseases should be handled no differently than other routine specimens. Adopting a universal precautions policy, which considers blood and other body fluids from all patients as potentially infective, is required.
Airborne Pathogens
Because of a global resurgence of TB, OSHA issued a statement in 1993 that the agency would enforce CDC Guidelines for Preventing the Transmission of Tuberculosis in Health Care Facilities. The purpose of the guidelines is to encourage early detection, isolation, and treatment of active cases. A TB exposure control program must be established, and risks to laboratory workers must be assessed. In 1997, a proposed standard (29 CFR 1910.1035, Tuberculosis) was issued by OSHA only to be withdrawn again when it was determined that existing CDC guidelines could be enforced by OSHA through its “general duty” clause and Respiratory Protection Standard. The CDC guidelines require the development of a tuberculosis infection control program by any facility involved in the diagnosis or treatment of cases of confirmed infectious TB. TB isolation areas with specific ventilation controls must be established in health care facilities. Those workers in high-risk areas may be required to wear a respirator for protection. All health care workers considered to be at risk must be screened for TB infection.
Other specific pathogens, including viruses, bacteria, and fungi, may be considered airborne transmission risks. Protective measures in the clinical laboratory generally involve work practice and engineering controls focused on prevention of aerosolization, containment/isolation, and respiratory protection of N-95 (filtration of 95% of particles >0.3 μm) or better.
Shipping
Clinical laboratories routinely ship regulated material. The U.S. Department of Transportation (DOT) and the International Air Transport Association (IATA) have specific requirements for carrying regulated materials. There are two types of specimen classifications. Known or suspect infectious specimens are labeled infectious substances if the pathogen can be readily transmitted to humans or animals. Diagnostic specimens are those tested as routine screening or for initial diagnosis. Each type of specimen has rules and packaging requirements. The DOT guidelines are found in the Code of Federal Regulations, Title 49, Subchapter C; IATA publishes its own manual, Dangerous Goods Regulations.
CHEMICAL SAFETY
Hazard Communication
In the August 1987 issue of the Federal Register, OSHA published the new Hazard Communication Standard (Right to Know Law, 29 CFR 1910.1200). The Right to Know Law was developed for employees who may be exposed to hazardous chemicals in the workplace. Employees must be informed of the health risks associated with those chemicals. The intent of the law is to ensure that health hazards are evaluated for all chemicals that are produced and that this information is relayed to employees.
To comply with the regulation, clinical laboratories must
- Plan and implement a written hazard communication program.
- Obtain SDSs for each hazardous compound present in the workplace and have the SDSs readily accessible to employees.
- Educate all employees annually on how to interpret chemical labels, SDSs, and health hazards of the chemicals and how to work safely with the chemicals.
- Maintain hazard warning labels on containers received or filled on-site.
In 2012, OSHA adopted significant changes to the Hazard Communication Standard to facilitate a standardization of international hazard communication programs. This new initiative was titled the Globally Harmonized System of Classification and Labelling of Chemicals, or GHS. The primary improvements to the program involved more specific criteria for classification of chemicals; a uniform system of chemical labeling, including intuitive pictographs; and, replacing the existing Material Safety Data Sheet (MSDS) program with the new SDS format. These changes were phased in over a 3-year period, with the final requirements effective in June of 2016.
Safety Data Sheet
The SDS is a major source of safety information for employees who may use hazardous materials in their occupations. Employers are responsible for obtaining the SDS from the chemical manufacturer or developing an SDS for each hazardous agent used in the workplace. The information contained in the SDS must follow a specific format, addressing each of the following 16 items:
- Section 1: Identification
- Section 2: Hazard identification
- Section 3: Ingredients information
- Section 4: First aid procedures
- Section 5: Fire-fighting procedures
- Section 6: Accidental-release measures
- Section 7: Handling and storage
- Section 8: Exposure controls and personal protection
- Section 9: Physical and chemical properties
- Section 10: Stability and reactivity
- Section 11: Toxicological information
- Section 12: Ecological information
- Section 13: Disposal considerations
- Section 14: Transport information
- Section 15: Regulatory information
- Section 16: Other information, including date of preparation or last revision
The SDS must provide the specific compound identity, together with all common names. All information sections must be completed, and the date that the SDS was printed must be indicated. Copies of the SDS must be readily accessible to employees during all shifts.
OSHA Laboratory Standard
Occupational Exposure to Hazardous Chemicals in Laboratories (29 CFR 1910.1450), also known as the laboratory standard, was enacted in May 1990 to provide laboratories with specific guidelines for handling hazardous chemicals. This OSHA standard requires each laboratory that uses hazardous chemicals to have a written chemical hygiene plan. This plan provides procedures and work practices for regulating and reducing exposure of laboratory personnel to hazardous chemicals. Hazardous chemicals are those that pose a physical or health hazard from acute or chronic exposure. Procedures describing how to protect employees against teratogens (substances that affect cellular development in a fetus or embryo), carcinogens, and other toxic chemicals must be described in the plan. Training in the use of hazardous chemicals must be provided to all employees and must include recognition of signs and symptoms of exposure, location of SDS, the chemical hygiene plan, and how to protect themselves against hazardous chemicals. A chemical hygiene officer must be designated for any laboratory using hazardous chemicals. The protocol must be reviewed annually and updated when regulations are modified or chemical inventory changes. Remember that practicing consistent and thorough hand washing is an essential component of preventative chemical hygiene.
Toxic Effects from Hazardous Substances
Toxic substances have the potential of producing deleterious effects (local or systemic) by direct chemical action or interference with the function of body systems. They can cause acute or chronic effects related to the duration of exposure (i.e., short-term, or single contact, versus long-term, or prolonged, repeated contact). Almost any substance, even the most benign seeming, can pose risk of damage to a worker’s lungs, skin, eyes, or mucous membranes following long- or short-term exposure and can be toxic in excess. Moreover, some chemicals are toxic at very low concentrations. Exposure to toxic agents can be through direct contact (absorption), inhalation, ingestion, or inoculation/injection.
In the clinical chemistry laboratory, personnel should be particularly aware of toxic vapors from chemical solvents, such as acetone, chloroform, methanol, or carbon tetrachloride, that do not give explicit sensory irritation warnings, as do bromide, ammonia, and formaldehyde. Air sampling or routine monitoring may be necessary to quantify dangerous levels. Mercury is another frequently disregarded source of poisonous vapors. It is highly volatile and toxic and is rapidly absorbed through the skin and respiratory tract. Mercury spill kits should be available in areas where mercury thermometers are used. Most laboratories are phasing out the use of mercury and mercury-containing compounds. Laboratories should have a policy on mercury reduction or elimination and a method for legally disposing off mercury. Several compounds, including formaldehyde and methylene chloride, have substance-specific OSHA standards, which require periodic monitoring of air concentrations. Laboratory engineering controls, PPE, and procedural controls must be adequate to protect employees from these substances.
Storage and Handling of Chemicals
To avoid accidents when handling chemicals, it is important to develop respect for all chemicals and to have a complete knowledge of their properties. This is particularly important when transporting, dispensing, or using chemicals that, when in contact with certain other chemicals, could result in the formation of substances that are toxic, flammable, or explosive. For example, acetic acid is incompatible with other acids such as chromic and nitric acid, carbon tetrachloride is incompatible with sodium, and flammable liquids are incompatible with hydrogen peroxide and nitric acid.
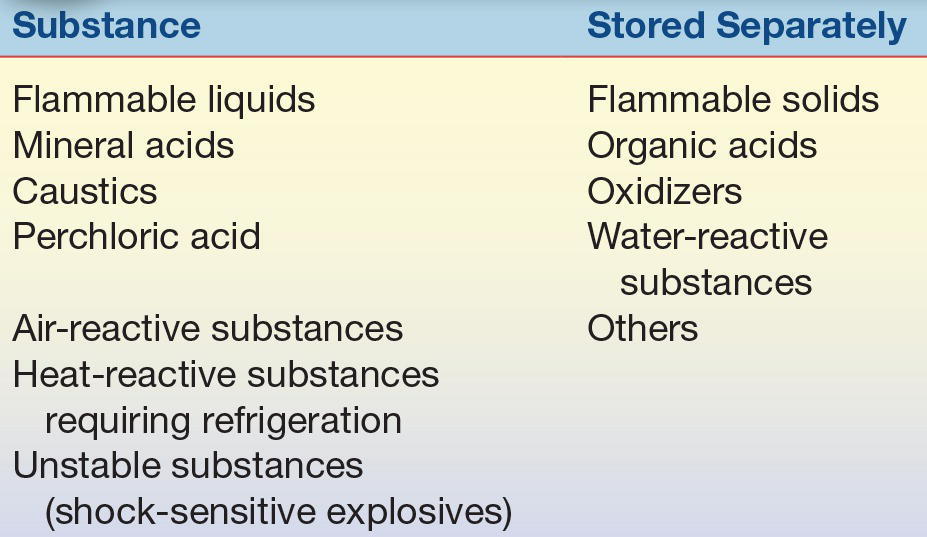
Arrangements for the storage of chemicals will depend on the quantities of chemicals needed and the nature or type of chemicals. Proper storage is essential to prevent and control laboratory fires and accidents. Ideally, the storeroom should be organized so that each class of chemicals is isolated in an area that is not used for routine work. An up-to-date inventory should be kept that indicates location of chemicals, minimum/maximum quantities required, and shelf life. Some chemicals deteriorate over time and become hazardous (e.g., many ethers and tetrahydrofuran form explosive peroxides). Storage should not be based solely on alphabetical order because incompatible chemicals may be stored next to each other and react chemically. They must be separated for storage, as shown in Table 2.2.
TABLE 2.2 Storage Requirements
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


