Kimura Disease
C. Cameron Yin, MD, PhD
Key Facts
Terminology
Chronic inflammatory disease that affects subcutaneous tissue and regional lymph nodes
Etiology/Pathogenesis
Unknown; infectious cause suspected
Clinical Issues
Mainly in young Asian males
Head & neck region
Nontender subcutaneous masses
Regional lymphadenopathy
Peripheral blood eosinophilia and elevated serum IgE
Benign clinical course; recurrence common
Microscopic Pathology
Skin
Typically located in deep subcutaneous tissue
Reactive follicles with prominent germinal centers
Eosinophilia and vascular hyperplasia
Lymph nodes
Hyperplastic follicles
Eosinophilia with eosinophilic microabscesses
Stromal and perivascular sclerosis
Ancillary Tests
Immunophenotypic studies
IgE deposits in germinal centers
Polytypic B cells and normal T cells
Top Differential Diagnoses
Angiolymphoid hyperplasia with eosinophilia
Langerhans cell histiocytosis
Dermatopathic lymphadenopathy
Parasitic infection
TERMINOLOGY
Abbreviations
Kimura disease (KD)
Synonyms
Kimura lymphadenopathy
Eosinophilic lymphogranuloma
Eosinophilic lymphoid follicular hyperplasia
Definitions
Rare chronic inflammatory disorder of unknown etiology
Commonly occurs in head & neck region and involves subcutaneous tissues and lymph nodes
Unrelated to angiolymphoid hyperplasia with eosinophilia (ALHE)
Historically, these entities were once considered to be the same
ETIOLOGY/PATHOGENESIS
Infectious Agents
History and histologic findings suggest infectious etiology
No definite pathogen has been identified
Other Proposed Causes
Allergy
Autoimmunity
CLINICAL ISSUES
Epidemiology
Age
Mainly occurs in young adults
Peak age of onset in 2nd and 3rd decades
Gender
Predominantly males
Ethnicity
Asians are most commonly afflicted
Named after T. Kimura from Japan who reported a case in 1948
Site
Usually in head & neck region
Involves deep subcutaneous tissues
Regional lymph nodes
Often involves major salivary glands
Parotid, submandibular
Presentation
Nontender masses in head & neck
Most often in periauricular region
Rarely patients have generalized lymphadenopathy
Systemic symptoms are uncommon
Laboratory Tests
Peripheral blood eosinophilia almost invariable
Elevated serum IgE level
Elevated erythrocyte sedimentation rate (ESR)
Imbalance between Th1 and Th2 cytokines
Increased TNF-α, IL-4, IL-5, IL-13, etc.
Natural History
Insidious onset
Slow-growing mass
Interval from onset of swelling to presentation may be several years
Often persists unchanged for years
Nephrotic syndrome may occur in up to 60% of patients
Some may have allergic conditions such as urticaria or eczema
Treatment
Adjuvant therapy
Surgical excision and steroid therapy are the most widely used treatments
Both have a high rate of recurrence
Advantages of surgical excision: Short treatment duration and provides tissue for histopathologic diagnosis
Local irradiation yields the best outcome
Not advocated in children due to the impact on growth and potential risk of malignancy
Prognosis
Indolent clinical course
Recurrence after excision and steroid therapy is common
IMAGE FINDINGS
General Features
Ultrasound, CT, or MR scans are useful for determining extent of disease
Combination of ultrasonography and MR has been shown to have high diagnostic value
Hypoechoic center and hyperechoic margin with enriched blood vessels on ultrasonography and Doppler
Hypointensity replaces normal hyperintense subcutaneous fat on MR
Lymph nodes are enlarged with well-defined outline
CT scan shows nonspecific findings
MICROSCOPIC PATHOLOGY
Histologic Features
Lymphoid infiltrate in deep subcutis
Formation of follicles with germinal centers
Accompanied by many eosinophils, plasma cells, and mast cells
Eosinophilic microabscesses can be present
Vascular hyperplasia
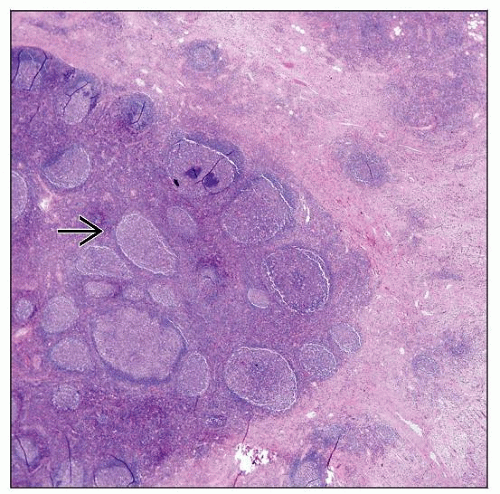
Lymph nodes show preserved but distorted overall architecture with
Hyperplastic follicles with well-formed germinal centers and mantle zones
Deposition of IgE in germinal centers forms hyaline proteinaceous material
Eosinophilia
Eosinophilic microabscesses and eosinophilic follicle lysis
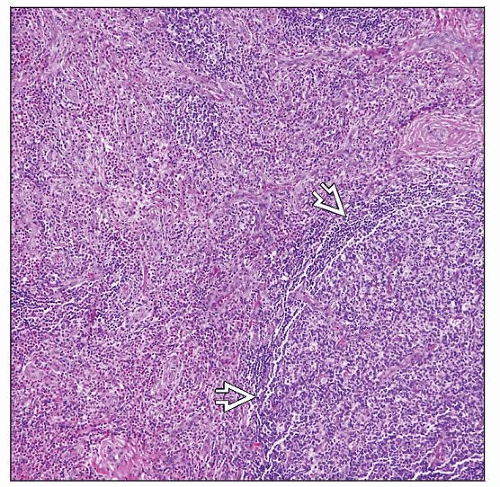
Involvement of perinodal soft tissues
Necrosis (±); usually not extensive
Vascular proliferation in interfollicular regions
Endothelial cells lack cuboidal/polygonal shape, e.g., “hobnail” or “tombstone” appearance (seen in ALHE)
Stromal and perivascular sclerosis
Cytologic Features
Fine-needle aspiration (FNA) yields polymorphous cell population with many eosinophils
Difficult to establish diagnosis of KD based on FNA findings alone
ANCILLARY TESTS
Immunohistochemistry
IgE deposits in the germinal centers can be shown by immunohistochemistry or immunofluorescence
Polytypic B cells and normal T cells
Molecular Genetics
No evidence of monoclonal gene rearrangements
No known translocations or oncogene abnormalities
No evidence of an infectious organism
DIFFERENTIAL DIAGNOSIS
Angiolymphoid Hyperplasia With Eosinophilia (ALHE)
ALHE has a number of other names
Epithelioid hemangioma is probably best name
Lesion is thought to be benign vascular neoplasm
Other names
Histiocytoid hemangioma
Pseudo- or atypical pyogenic granuloma
Inflammatory angiomatous nodule
Intravenous atypical vascular proliferation
Occurs more often in
Caucasians
Young to middle-aged adults
Presents as multiple papules or nodules
Usually occurs in head and neck region
Common around ear
Peripheral blood eosinophilia occurs in ˜ 15% of ALHE patients
Histologic findings of ALHE differ from Kimura disease as follows
Located in superficial dermis
Lesion has low-power lobular pattern of capillary or medium-sized blood vessels
Hypertrophic cuboidal/polygonal endothelial cells
Protrude into or occlude vascular lumina
Described as “hobnail” or “tombstone” appearance
Lesion can be located within large blood vessel
No lymph node involvement
Local recurrence can occur in ˜ 33% of patients
Langerhans Cell Histiocytosis
Young children, adolescents, and young adults
Can involve lymph nodes or extranodal sites
However, deep subcutis is unlikely site of involvement (in contrast with KD)
Lymph nodes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree