Irritable Bowel Syndrome and Diverticular Disease1
Lauren Schwartz
Carol E. Semrad
1Abbreviations: CI, confidence interval; COLAP, colonoscopic allergen provocation; FOD-MAPs, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; HLA, human leukocyte antigen; IBS, irritable bowel syndrome; IBS-C, IBS with constipation predominance; IBS-D, IBS with diarrhea predominance; IBS-M, IBS with mixed constipation and diarrhea; Ig, immunoglobulin; RAST, radioallergosorbent test; RR, relative risk.
IRRITABLE BOWEL SYNDROME
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder characterized by a combination of abdominal pain or discomfort and altered bowel habits over a period of at least 3 months that is not explained by structural, histologic, or biochemical abnormalities (1). IBS is a common condition, affecting 7% to 10% of the population worldwide and greatly affects health care use (1). It is considered a functional bowel disorder. Individuals are classified into one of three subtypes: IBS with constipation predominance (IBS-C), IBS with diarrhea predominance (IBS-D), and IBS with mixed constipation and diarrhea (IBS-M). The pathophysiology of IBS is unknown. The current disease model focuses on the brain-gut axis, a bidirectional pathway of communication between the central and enteric nervous systems. This pathway is influenced by various physiologic and psychosocial factors that contribute to heightened visceral sensitivity and disordered defecation. Dietary factors, changes in the intestinal microbiome, inflammation, altered motility and sensation, anxiety, and depression are among the variables believed to impinge on this pathway and exacerbate IBS symptoms (1).
Diet and Irritable Bowel Syndrome Symptoms
Up to two thirds of patients who suffer from IBS consider their gastrointestinal symptoms to be food related and modify their diets to avoid symptom triggers. Among these patients, approximately 12% overly restrict their intake and consume inadequate or unbalanced diets (2, 3). Commonly identified culprit foods include milk products, raw vegetables (especially onions, cabbage, and beans), fatty foods, spicy foods, coffee, and alcohol. These items have been linked to excessive gas-bloat symptoms and abdominal pain, followed by dyspepsia and loose stools (2). In most instances, reported food intolerances are not substantiated by formal testing for food allergies, malabsorption, or celiac disease (3). Perception of food intolerance is higher in the IBS population (60% to 70%) than in the general population (20% to 25%) (3, 4, 5). This difference is not explained by differences in food consumption. Individuals with functional gastrointestinal disorders consume similar proportions of foods containing wheat, lactose, caffeine, fructose, alcohol, and bioactive substances (e.g., serotonin, tryptophan) as case controls (6).
Diet and Irritable Bowel Syndrome Pathophysiology
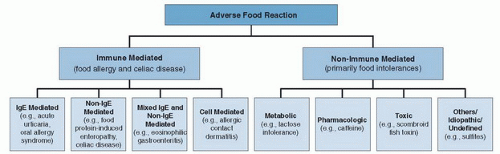
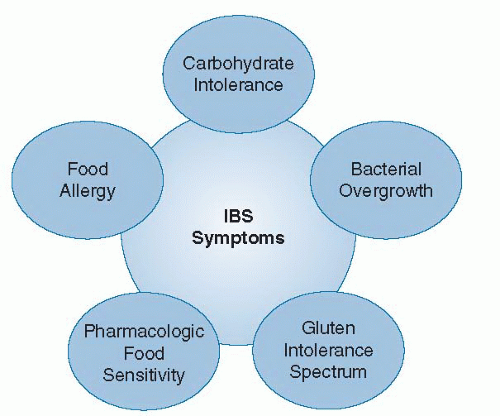
Adverse reactions to foods are classified as either immune mediated, such as food allergy, or nonimmune mediated, such as a specific food intolerance. A general schema of adverse food reactions and associated mechanisms is depicted in Figure 80.1 (7). These varied mechanisms cause gastrointestinal symptoms compatible with IBS (Fig. 80.2).
Immune-Mediated Conditions
Food Allergy
The role of food allergy in the pathogenesis of IBS is controversial. Although data from dietary elimination and food challenge studies support the role of dietary triggers in a subgroup of IBS patients, correlation with allergy testing has been inconsistent. Only one out of three food elimination-challenge studies performing skin-prick or
radioallergosorbent test (RAST) detected a positive correlation between diagnostics and dietary challenge (8, 9, 10, 11). Studies of oral cromolyn sodium, an inhibitor of mast cell degranulation, demonstrated efficacy in modulating IBS symptoms. In a large multicenter study of patients with diarrhea-predominant IBS, cromolyn sodium was as effective as an elimination diet in improving symptoms (67% versus 60%) (12). Treatment response was greater in patients with a positive skin-prick test to food allergens, a finding suggesting that a subset of patients with IBS had allergy-related symptoms.
radioallergosorbent test (RAST) detected a positive correlation between diagnostics and dietary challenge (8, 9, 10, 11). Studies of oral cromolyn sodium, an inhibitor of mast cell degranulation, demonstrated efficacy in modulating IBS symptoms. In a large multicenter study of patients with diarrhea-predominant IBS, cromolyn sodium was as effective as an elimination diet in improving symptoms (67% versus 60%) (12). Treatment response was greater in patients with a positive skin-prick test to food allergens, a finding suggesting that a subset of patients with IBS had allergy-related symptoms.
Novel diagnostic tests may help clarify the role of food allergy in IBS. Increased fecal immunoglobulin E (IgE) levels have been reported in patients with food hypersensitivity based on history, skin-prick test, and RAST test, but not in healthy controls (13). Of the patients with IBS included in the study, 68% were found to have detectable fecal IgE fragments. The colonoscopic allergen provocation (COLAP) test also demonstrated hypersensitivity reactions at the level of the intestine that are not detectable by skin tests or serum IgE levels. Injection of food extracts into the colonic submucosa produced weal and flare responses in 74% of patients with IBS and in no healthy controls. Biopsies of these sites confirmed mast cell and eosinophil activation, and elimination of suspected food agents resulted in symptom improvement in the majority of COLAP-positive patients (8, 14). An in vitro assay that quantifies basophil activation by food antigens based on CD63 expression identified food hypersensitivity in patients with IBS with high levels of sensitivity, specificity, and accuracy (15). IgG-mediated hypersensitivity has been proposed as an alternative mechanism of food allergy in IBS patients. Although IgG antibodies are commonly considered a physiologic response to food antigen exposure, higher IgG4 levels to common food items have been detected in IBS subjects (16). Greater reductions in IBS symptom scores were observed following an elimination diet based on IgG antibodies as compared with scores following a sham diet (17).
Celiac Disease
Approximately 75% of patients with celiac disease present with gastrointestinal symptoms that overlap with IBS, including abdominal pain, bloating, and altered bowel habits (18). In the United States, the prevalence of celiac disease in the general population approaches 1%, but it may be as high as 4% in the IBS population (19, 20). A fourfold higher risk of histologically confirmed celiac disease exists among patients with IBS when compared with controls (20). Conversely, 20% of patients with celiac disease fulfilled Rome III criteria for IBS compared with 5% of controls (21). The exact relationship between celiac disease and IBS is uncertain. IBS possibly represents a misdiagnosis of celiac disease, or celiac disease may coexist with IBS. Other conditions such as inflammatory bowel disease, gastroenteritides, and small intestinal bacterial overgrowth have been associated with IBS, to suggest IBS is a downstream effect of various forms of mucosal inflammation (22). The American College of Gastroenterology IBS Task Force currently recommends routine serologic screening for celiac disease in patients with IBS-D and IBS-M (1). This approach is cost effective when the prevalence of celiac disease in a population is 3% or higher (23, 24).
More controversial is the linkage of gluten sensitivity to IBS. Gluten sensitivity is an as yet ill-defined condition characterized by gastrointestinal symptoms exacerbated by gluten ingestion and alleviated by gluten exclusion, in the absence of serologic and histologic evidence of celiac disease. In a study of patients with IBS-D with negative celiac serologic test results, 35% were human leukocyte antigen (HLA)-DQ2 positive, 23% had increased intraepithelial lymphocytes, and 30% had celiac-related antibodies in duodenal aspirates (25). A gluten-free diet in individuals with IBS-D who were HLA-DQ2 positive or with positive intestinal aspirate antibodies resulted in significant improvement in diarrheal symptoms when compared with those without these features (25, 26). However, this may have been related to decreased fiber in the diet. In transgenic mice expressing HLA-DQ8, T-cell proliferation is enhanced without generating villous atrophy, and acetylcholine release in the myenteric plexus is increased on gluten exposure (27, 28). These findings offer a potential inflammatory and neuromotor basis for IBS-type symptoms.
Food Intolerance
Food intolerance refers to adverse food reactions resulting from various nonimmune mechanisms, including direct effects of toxins, pharmacologic agents in foods (e.g., caffeine, tyramine), malabsorption caused by host enzyme or transport deficiency (e.g., lactase, fructose), and idiosyncratic reactions.
Carbohydrate Malabsorption
Carbohydrate malabsorption of lactose, fructose, and sugar alcohols (e.g., sorbitol, xylitol) has been implicated as an underlying cause or trigger for IBS symptoms. The reported prevalence of lactose intolerance in IBS is highly variable and ranges from 17% to 82%, with most studies demonstrating rates of 25% to 35% in US and European populations (29, 30, 31, 32, 33, 34, 35). Patients with IBS have higher symptom scores from carbohydrate malabsorption compared with controls who do not have IBS, perhaps related to underlying visceral hyperalgesia (36). Lactose breath testing is recommended in subjects with IBS with diarrhea and bloating who lack a clear diagnosis based on food diary data alone (1). A positive breath test result without symptoms suggests lactose maldigestion of uncertain significance, whereas a positive test result accompanied by symptoms suggests true lactose intolerance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree