Diabetes mellitus (DM) is a metabolic disorder characterized primarily by abnormally high levels of glucose (sugar) in the blood, with other multiple metabolic abnormalities often present. It is one of the most prominent epidemics worldwide. DM afflicts 25.8 million people of all ages in the United States and more than 220 million in the world, with the rate of DM-related deaths predicted to double between 2005 and 2030 (
1,
2,
3,
4). DM and its complications have serious health and economic impacts. DM is the seventh leading cause of death in the United States (
5). It is the leading cause of blindness among working-age adults (
6), nontraumatic amputations (
7), end stage renal disease and dialysis (
3,
8), and peripheral neuropathy (
9,
10). Further, having DM significantly increases the risk for heart disease and stroke (
11,
12,
13). In particular, with the growing presence of decreased physical activity and the increased availability of calorically dense foods, the prevalence of DM is increasing worldwide, especially in developing countries and in adolescents, as with obesity.
As demonstrated by the Diabetes Prevention Program (
14), the most successful strategy in the treatment and prevention of DM is lifestyle modification. Because most serum glucose depends on dietary intake, medical nutrition therapy (MNT) remains a cornerstone in the treatment of DM and continues to prove quite effective in the overall management of the disease (
15). Physical activity is also very important in achieving lifestyle changes. Self-monitoring of blood glucose levels and appropriate adjustments of food intake, exercise, and medications can facilitate optimal glycemic control (
16). In all cases, an integrated health care team is essential for persons with DM to maintain quality of life and longevity.
HISTORICAL OVERVIEW
Classic symptoms of DM, such as polyuria and polydipsia, have been observed and treated with dietary interventions for more than thousands of years in early Egyptian, Indian, and Greek civilizations. One of the earliest reports, the Ebers Papyrus written in 1500 BC (discovered in a tomb in the Thebes region in southern Egypt in 1862 and named after the Egyptologist Geary Ebers), describes a common symptom, polyuria, of the “sugar disease” (
17). Egyptians suggested various dietary remedies for this syndrome, including a diet of beer, fruits, grains, and
honey (
17). Early Indians described DM-like symptoms and recommended the freshly harvested cereals and bituminous preparations containing benzoates and silica as a remedy for DM (
18). Aretaeus of Cappadocia (81 to 138 AD) first used the Greek word “diabetes,” literally meaning “to run through” or siphon. He described the disease as the “melting down of flesh and limbs into the urine.” He concluded that diabetes was a disease of the stomach and should be treated with milk, gruel, cereals, fruits, and sweet wines. Milk, water, wine, and beer were also used as the main fluids to relieve excessive thirst until the second century AD, when diabetes was thought to be a disease of the kidneys (
19). A London physician, Thomas Willis, added “mellitus,” meaning honeylike, after noticing the sweet taste of urine.
In the late 1700s, a French physician began prescribing undernutrition or semistarvation diets, interspersed with frequent periods of fasting, for patients with DM (
19). In the early 1900s, Dr. Frederick M. Allen, in the United States, developed his starvation diet and was one of the first to customize or “individualize” diets to his patients’ preference while still providing only 1000 calories a day. Although many of his patients were malnourished, Allen is credited with helping many survive before the introduction of insulin therapy in 1921 (
19). The discovery of insulin in the 1920s dramatically increased survival for those afflicted with DM. However, diet recommendations still remained controversial. Similar to the present day, whereas some physicians were proponents of highcarbohydrate and low-fat diets, others were proponents of low-carbohydrate, high-protein, and high-fat diets (
19). The second approach has generally fallen out of favor because of the increased risk of heart disease.
In 1994, shortly after clinical findings from the Diabetes Control and Complications Trial (DCCT) were published, the American Diabetes Association (ADA) published a revised set of nutrition guidelines, to refocus on an “individualized approach to nutrition self-management that is appropriate for the personal life-style and diabetes management goals of the individual with diabetes” (
20). Although the core emphasis of these nutrition guidelines remains, the guidelines have continued to evolve.
CLASSIFICATION
The classification of DM has moved away from a system based, in large part, on the type of pharmacologic treatment to a system based on disease origin (
21). The terms
insulin-dependent DM (IDDM) and
non-insulindependent DM (NIDDM) should no longer be used (
21,
22,
23), because some patients who initially have type 2 NIDDM can eventually develop insulin dependence. DM is a clinically heterogeneous group of disorders that share hyperglycemia in common and that result from insulin insufficiency, insulin resistance, or both (
24). An appropriate system of classification is important for the clinical management of DM (
21). No systematic categorization had been generally accepted until the National Diabetes Data Group (NDDG) classification system was published in 1979 (
21,
22). The World Health Organization (WHO) Expert Committee on Diabetes in 1980 and, later, the WHO Study Group on Diabetes Mellitus endorsed the recommendations of the NDDG (
21,
23). Currently, the ADA and the WHO classify DM into four main clinical classifications: type 1, type 2, other specific types of DM, and gestational diabetes mellitus (GDM) (
25). Most cases are type 1 or type 2 DM (
24).
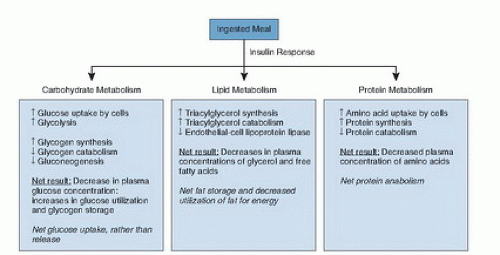
Type 1 DM is characterized by absolute insulin deficiency caused by autoimmune-mediated destruction of the pancreatic β cells, and it accounts for 5% of all cases. Type 2 DM, which accounts for about 90% to 95% of cases, is characterized by two primary defects: insulin resistance (diminished peripheral tissue sensitivity to insulin) and relatively impaired β-cell function (delayed or inadequate insulin release). GDM is defined as abnormal glucose status that is first recognized during pregnancy. The other types (i.e., unusual genetic forms or secondary causes of DM) account for the remaining cases in the United States. The origin and classification can be found in
Table 61.1.
EPIDEMIOLOGY
The worldwide prevalence of DM has risen dramatically since the 1990s. DM is one of the most common chronic diseases in most countries, and it continues to increase in number and significance as changing lifestyles lead to reduced physical activity, increased intake of energydense foods, and increased obesity (
26,
27). An estimated 25.8 million people are affected by DM (7 million of these people have undiagnosed cases), and 1.9 million people 20 years old or older were newly diagnosed with DM in 2010 in the United States (
3). This number will almost double to 44.1 million by 2035 (
26). Along with the United States, the global epidemic is also growing. Worldwide, 220 million people have DM; and the overall total predicted increase in numbers with DM from 2010 to 2030 is 54% at an annual growth of 2.2%, nearly twice the annual growth of the total world adult population (
27). Developing countries have an anticipated disproportionate 69% increase compared with a 20% increase in developed countries between 2010 and 2030 (
27). Thirtysix percent of the anticipated global increase of DM (154 million people) is projected to occur in India and China alone (
27).
The economic impact of DM and its complications is great. Research suggests that the total cost of diabetes in 2007 for the United States was $174 billion, including $116 billion in excess medical expenditures and $58 billion in reduced national productivity. Medical costs attributed to DM included $27 billion for care to treat DM directly, $58 billion to treat DM-related chronic complications, and $31 billion in general medical costs. Indirect costs include absenteeism from work, reduced
work productivity, unemployment from disease-related comorbidity, and lost productive capacity from early mortality (
26,
28,
29,
30). Further, total estimated global health care expenditures on DM were $376 billion in 2010 and are $490 billion in 2030 (
31).
Considerable geographic and genetic variation is noted in the incidence of both type 1 and type 2 DM. The global age-adjusted incidence of type 1 DM ranges between the lowest incidence (0.1 per 100,000/year) in China and Venezuela to the highest incidence (40.9 per 100,000/ year) Finland and Sardinia (Italy) (
32,
33). This incidence is increasing 3% to 4% per year worldwide, a finding possibly suggesting an environmental contribution to the disease (
32). Soltesz et al (
34) analyzed numerous epidemiologic studies and found environmental risk factors early in life to be possibly contributors to the increasing incidence of type 1 DM, including enteroviral infections in pregnant women (
35,
36,
37,
38,
39), older maternal age (
39,
40,
41), preeclampsia (
42), cesarean section delivery (
41,
42), increased birth weight (
43), early introduction of cow’s milk proteins, and an increased rate of postnatal growth (weight and height) (
44,
45). Vitamin D supplementation may be protective (
46). Viruses may initiate autoimmunity toward the β cell, whereas other exposures may overload the β cell and accelerate DM development (
34). Assuming that present trends in Europe continue, scientists predict doubling of new cases of type 1 DM in European children less than 5 years of age between 2005 and 2020 and a 70% rise in prevalent cases in patients less than 15 years of age (
47).
Type 2 DM affects 90% to 95% of people with DM around the world, and it is associated with excess body weight and decreased physical inactivity. The prevalence of type 2 DM in the United States is increasing as the obesity epidemic is exploding. The onset is usually in older adults more than 35 years old, although type 2 DM is occurring more often in youth. Risk factors include physical inactivity (exercising less than three times per week), high-risk ethnicity (e.g., African-American, Latin American, Native American, Asian American, or Pacific Islander), delivery of a baby weighing more than 9 lb (4 kg) or a diagnosis of GDM, hypertension, high-density lipoprotein cholesterol (HDL-C) levels less than 35 mg/dL (0.90 mmol/L) and/or triglyceride levels higher than 250 mg/dL (2.82 mmol/L), polycystic ovarian syndrome, previously identified impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), clinical conditions associated with insulin resistance, and history of cardiovascular disease (CVD). Having a firstdegree relative with type 2 DM (i.e., a parent or sibling) increases the risk of DM by 40%. Abdominal obesity
confers higher risk, and waist circumference cutoffs may vary by ethnicity (
48).
Some other risk factors for type 2 DM include the following: older age (
1); greater parity (
49); low (versus moderate) alcohol use (
50); cigarette smoking (
51), as well as (transiently) stopping smoking (
52); stress (
53); low socioeconomic status, particularly among Latin Americans and African-Americans (
54,
55); diet (i.e., high-fat, lowfiber, Western diet) (
56); low magnesium intake (
57); and soda consumption (
58). Strong urbanization trends also associated with type 2 DM are clearly depicted in the following two epidemiologic cases. For example, in the Pacific island of Nauru, Pima Indians took on a more Western lifestyle leading to more obesity, and their DM incidence increased from nearly 0% to about 50% (
59). In India, Asian Indians living in rural communities had a DM prevalence of 2%, which increased to 10% after they moved to an urban environment (
60).
Prediabetes or intermediate DM is an emerging concern. Persons with prediabetes have an increased risk of developing type 2 DM, CVDs, and microvascular diseases such as peripheral neuropathy. In 2005 to 2008, based on fasting glucose or hemoglobin A1c levels, 35% of US adults 20 years old or older had prediabetes (
61). According to the Centers for Disease Control and Prevention (CDC), applying this percentage to the entire US population in 2010 yields an estimated 79 million US residents 20 years old or older with prediabetes (
3).
GDM affects an estimated 170,000 (1% to 14%) pregnancies each year in the United States (
62). For those women who are diagnosed with GDM, 30% to 50% will have recurrent GDM in a future pregnancy (
63,
64). Of particular concern, up to 50% of women with GDM will develop type 2 DM in the 5 to 10 years after delivery of their baby (
62). In a metaanalysis, Bellamy et al reported that GDM corresponded to a 7.4-fold increased risk for developing future type 2 DM (
63).