Chlorhexidine digluconate solution
0.53 g
Acetic Acid (6 %) B.P.
q.s.
Water for injections
ad 100 mL
Bladder irrigations with the purpose of eliminating bladder stones, or preventing the formation of bladder stones are another group. As well as a mechanical cleaning action a chemical reaction is necessary to dissolve the stone or slivers of stone, which consist of calcium and magnesium compounds. Therefore complexing agents such as citrate and edetate are added.
With transurethral prostate resection the bladder has to be irrigated with large quantities of solution to ensure that the view during the operation is not reduced by the presence of blood.
There are also solutions for intravesical use (use in the bladder) that have to act locally in disorders of the bladder and are not meant to irrigate. Examples are aluminium for bladder bleedings, antineoplastics for bladder cancer and oxybutinin for urge incontinence.
Eye lotions (irrigations for the eye) are discussed in Sect. 10.6.2. Vaginal solutions that don’t need to be sterile are discussed in Sect. 11.12. Whole bowel irrigations are used before diagnostic examination. Those solutions for oral or rectal use are not described in this chapter.
14.1.2 Dialysis Solutions
Dialysis serves the purpose of removing waste products from the body when the kidneys cannot do this anymore. There are various forms of dialysis with their own characteristics.
With haemodialysis the blood passes an artificial kidney via extracorporeal circulation. The artificial kidney removes waste products and fluid. This happens mainly by osmosis (diffusion) via a semipermeable membrane, and partly by convection that is via the transport of water and waste products forced by pressure (ultrafiltration).
With haemofiltration the transport of fluid and waste products under pressure (ultrafiltration) is the main process. The pores in these membranes are a little larger than those at haemodialysis and the filtered fluid has to be substituted. The advantage is that waste products with a larger molecular weight are also removed, thus better resembling the filtration process in the normal kidney. This may have a favourable effect on cardiovascular health [2].
In practice a combination of dialysis and filtration is used, haemodiafiltration. Haemodiafiltration (HDF) is a combination of diffusion and convection. Diffusion is mainly effective for the removal of small waste molecules such as urea and creatinine. Larger molecules, for example beta-2-microglobuline, may only be removed from the blood by convection. For sufficient convective transport per HDF treatment an equivalent of 60 L of plasma is filtrated. At the same time the same volume is given back to the patient in the form of substitution solution. The substitution solution enters the circulation of the patient. This is the same process as the administration of an infusion, which is why some European Inspectorates regards solutions for HDF as parenterals.
With peritoneal dialysis the peritoneum, which is well supplied with blood vessels, functions as a semipermeable membrane. Peritoneal dialysis solutions are sterile hyper-osmotic solutions. These solutions withdraw water from the blood through the peritoneum. The transport of small ions takes place at the same time.
Peritoneal dialysis is often self-administered, the solution has to be changed four to five times a day. This is known as continuous ambulant peritoneal dialysis (CAPD).
Another method is automatic peritoneal dialysis (APD), whereby a machine performs the irrigation during the night. There are advantages and disadvantages to haemodialysis and peritoneal dialysis.
Peritoneal dialysis gives the patient more freedom than haemodialysis, but requires a suitable space at home and appropriate skills. The patient may gain weight from these glucose containing solutions.
Haemodialysis almost always takes place in a dialysis centre, mostly three times a week during several hours or during night hours. It may in principle also be done at home, but hygiene and precision are very much required. Haemodialysis has the disadvantage that the patient usually is not allowed to drink much fluid and is restricted to a specific diet.
14.2 Definitions
The Ph. Eur. describes irrigations (Preparations for irrigation) as follows [3]: “Preparations for irrigation are sterile, aqueous, large-volume preparations intended to be used for irrigation of body cavities, wounds and surfaces, for example during surgical procedures. Preparations for irrigation are either solutions prepared by dissolving one or more active substances, electrolytes or osmotically active substances in water complying with the requirements for Water for injections (0169) or they consist of such water alone. In the latter case, the preparation may be labelled as ‘water for irrigation’. Irrigation solutions are usually adjusted to make the preparation isotonic with respect to blood.”
This definition is valid for irrigations for the bladder, but also for other solutions for intravesical use.
Haemodialysis solutions (Solutions for haemodialysis) according to the Ph. Eur. are: “Solutions of electrolytes in a concentration close to the electrolyte composition of plasma (…). Because of the large volumes used, haemodialysis solutions are usually prepared by diluting a concentrated solution with water of suitable quality (see the monograph Haemodialysis solutions, concentrated, water for diluting (1167)), using for example an automatic dosing device.” They are prepared in a way that ensures a contamination level as low as possible. Haemodialysis solutions do not have to be sterile, they are not in direct contact with the blood. However, new dialysis membranes with larger pores may entail a considerable amount of back-filtration which in the near future probably will result in stricter requirements for these haemodialysis solutions.
Haemofiltration and haemodiafiltration solutions (Solutions for haemofiltration and for haemodiafiltration) according to the Ph. Eur. are: “Preparations for parenteral administration containing electrolytes with a concentration close to the electrolytic composition of plasma.” Because they are considered as parenterals they must be sterile (that is, they must comply with the test for sterility as described in the Ph. Eur.).
Peritoneal dialysis solutions (Solutions for peritoneal dialysis) according to the Ph. Eur. are: “Preparations for intraperitoneal use containing electrolytes with a concentration close to the electrolytic composition of plasma.” Although it is not mentioned in the description that they have to be sterile, they must comply with the test for sterility.
14.3 Biopharmaceutics
Solutions for intravesical use are meant to remain in the bladder for a longer period and to exert a pharmacological effect, for example oxybutinin bladder irrigation for urine incontinence. The addition of bioadhesive polymers, for example hypromellose, may prolong the effect.
Solutions for CAPD are inserted into the abdomen via a catheter (Fig. 14.1).
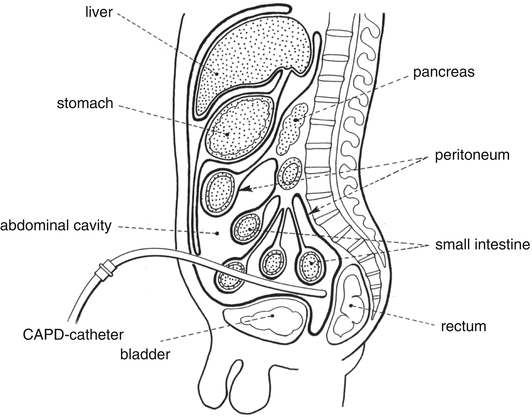

Fig. 14.1
Continuous ambulant peritoneal dialysis. Source: Recepteerkunde 2009, ©KNMP
The abdomen is surrounded by the peritoneum, an endothelial, single cellular layer that functions as dialysis membrane for water and small molecules. By using a hyper-osmotic solution for peritoneal dialysis water and small molecules are withdrawn from the blood. After several hours the fluid is rinsed out and replaced with new CAPD solution. Solutions for peritoneal dialysis are made iso to hyper-osmotic with glucose to remove water from the body. The glucose concentration is 1.36–4.25 %. The absorption of glucose from the dialysis solution may therefore be considerable.
14.4 Product Formulation
14.4.1 Irrigations
Irrigations are aqueous solutions. Active substances to be added have to be sufficiently water soluble.
14.4.1.1 Bacterial Endotoxins
The Ph. Eur. requires irrigations to contain maximally 0.5 IU/mL bacterial endotoxins. This requirement, therefore, is indicative as to how irrigations should be prepared. For irrigations for superficial wounds the need for the absence of bacterial endotoxins may be questioned. Apart from the situation with deep surgical wounds, for example during major surgery, the absorption of bacterial endotoxins is unlikely.
Endotoxin-free solutions require the use of bacterial endotoxin free starting materials. For dialysis solutions separate requirements exist, see Sect. 14.4.2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


