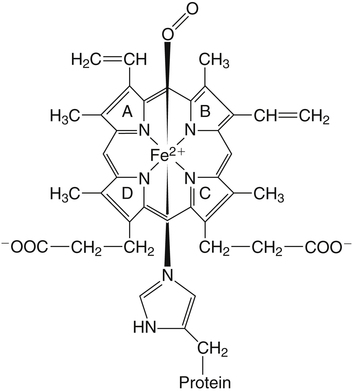
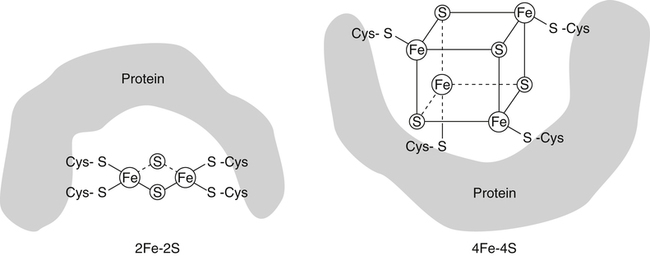
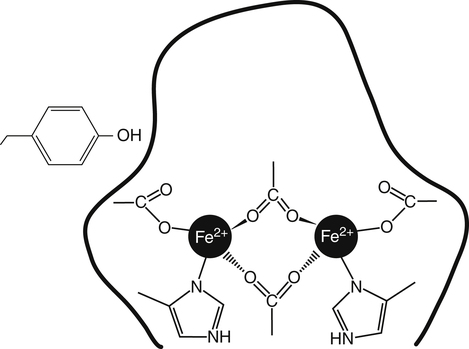
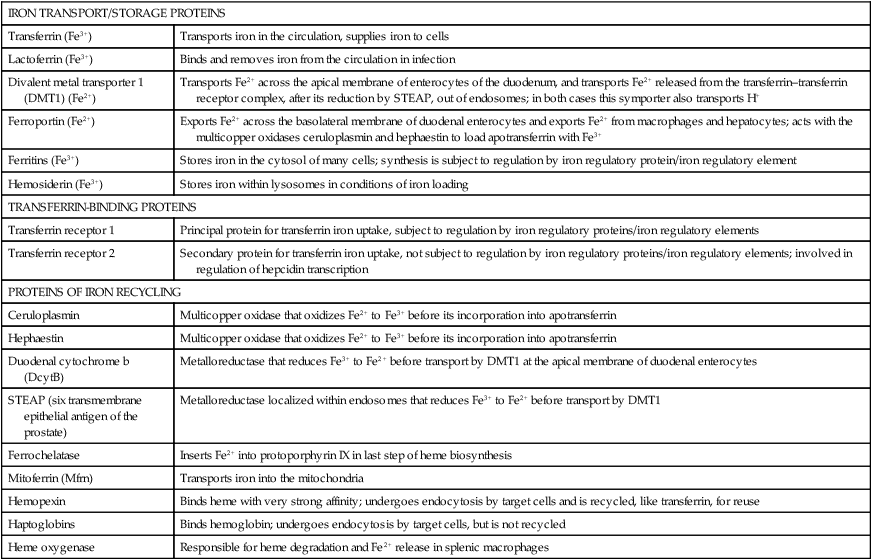
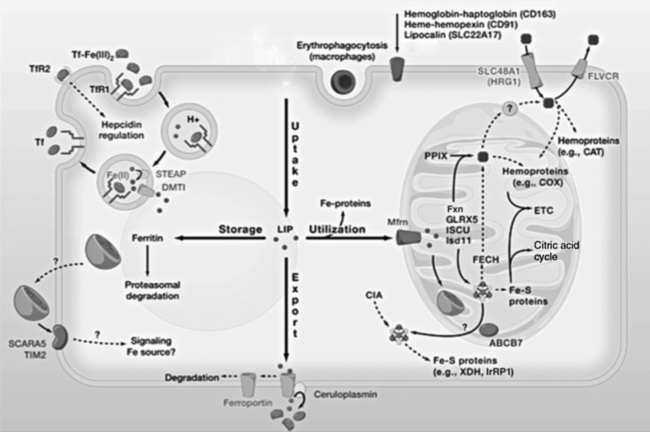
Iron is an almost ubiquitous requirement for life in any form, and for humankind it is quite simply indispensable. Iron-related disorders leading to either exhaustion or overloading of iron stores are extremely common in all parts of the world. Iron deficiency as a cause of anemia has been known for centuries. Iron overload, whether due to failure to prevent unneeded dietary iron entering the circulation (genetic hemochromatosis) or as a long-term consequence of repeated blood transfusions, is also a well-established clinical condition. This unusual susceptibility of humans to iron-related disorders is due to their severely limited capacity both to excrete iron and to absorb dietary iron when compared to other mammals. It follows therefore that, as was originally suggested by McCance and Widdowson (1937), iron balance is primarily determined by iron absorption. Iron is a constituent of numerous proteins, particularly those involved in the transport and metabolism of oxygen, as can be seen from the examples in Box 36-1. Iron proteins can be classified according to the coordination chemistry of their iron: heme proteins, iron-sulfur (Fe-S) proteins, and nonheme, non–iron-sulfur proteins. This latter group includes proteins that contain single Fe atoms and diiron μ-oxygen-bridged centers as well as proteins involved in iron transport and storage. Iron most often has a coordination number of six, which gives octahedral stereochemistry; however, tetracoordinate iron (e.g., Fe-S clusters) and five-coordinate complexes are also occasionally found (Crichton, 2008, 2009). The iron in heme proteins is present within a porphyrin, which consists of four pyrrole rings linked by methene bridges; in most hemoproteins the porphyrin is protoporphyrin IX, with four methyl, two vinyl, and two propionyl substituents as shown in Figure 36-1. Heme Fe is always coordinated to the four nitrogen atoms of the tetrapyrrole structure; the fifth, and in some cases the sixth, coordination position is occupied by amino acid residues of the protein. Heme itself is Fe2+–protoporphyrin IX, whereas the corresponding Fe3+–protoporphyrin IX complex is defined as hemin. In some heme proteins, such as cytochrome c, the porphyrin is covalently linked to amino acid residues of the protein. Heme proteins are involved in a great number of functions (see Box 36-1). The heme proteins include the oxygen carriers hemoglobin and myoglobin and many heme-containing enzymes such as cytochrome oxidase, peroxidases, catalases, and cytochrome P450s, which are involved in the activation of O2 or in the metabolism of peroxides (ROOHs). The iron atoms of these heme proteins possess an unoccupied sixth coordination site. This sixth site serves as the binding site for O2, other inorganic ligands (e.g., nitric oxide [NO]), or organic ligands (e.g., through carbon–iron bonds in cytochrome P450s) (Crichton, 2009). Examples of such heme-containing enzymes are listed in Box 36-1 and include monooxygenases (hydroxylases), dioxygenases, and peroxidases. In contrast, in most of the electron transporting cytochromes, the sixth coordinate position of the Fe2+ of the heme is occupied by an amino acid residue of the protein (often a histidine or methionine residue). In contrast to cytochrome a3 and cytochrome P450, which both bind oxygen and transfer electrons, most cytochromes only transfer electrons, with the iron in the heme alternating between the Fe2+ and Fe3+ states. The most common types of iron-sulfur centers are 2Fe-2S and 4Fe-4S clusters, which consist of equal numbers of iron and sulfide ions; the iron atoms of each cluster are also coordinated to the sulfur atoms of four cysteine residues in the protein, as shown in Figure 36-2. When both the sulfide atoms and the cysteine residues are considered, one can see that each Fe atom is tetrahedrally coordinated by four sulfur atoms. Most Fe-S cluster proteins are involved in electron transfer reactions. These include proteins of the mitochondrial electron transport chain as well as various mini–electron transport systems, such as the adrenodoxin (i.e., ferredoxin 1) component of mitochondrial cytochrome P450 systems. However, a number of Fe-S proteins have functions other than electron transfer, including catalysis and acting as biological sensors. The mitochondrial enzyme aconitase contains a 4Fe-4S center that acts as a Lewis acid in the dehydration reaction in which citrate is converted to isocitrate. As discussed later in this chapter, there is also a cytosolic aconitase, which is known as iron regulatory protein 1 (IrRP1) that acts as a biosensor for iron levels within the cell. A number of enzymes contain a single Fe atom, which is coordinately bound to imidazole (histidine) or carboxylate (glutamate and aspartate) ligands on the protein; these enzymes are involved in reactions that use O2 as substrate. The aromatic amino acid hydroxylases, which require tetrahydrobiopterin as a cofactor, constitute the first subgroup. A second subgroup requires a keto acid substrate (usually α-ketoglutarate) and a reducing agent (ascorbate). In these reactions, one of the oxygen atoms from O2 is transferred to the substrate, which is hydroxylated, and the other oxygen atom is transferred to the keto acid cosubstrate, which undergoes oxidative decarboxylation. (See Chapter 27 for examples and an explanation of the role of ascorbic acid in these reactions.) Finally there are some other mononuclear iron-containing enzymes, which add both oxygen atoms from O2 to a single substrate; these dioxygenases include the 15-lipoxygenase involved in eicosanoid synthesis and cysteine dioxygenase involved in cysteine catabolism and taurine synthesis (see Chapters 14 and 18). A family of proteins containing carboxylate-bridged dinuclear iron sites is involved in functions as diverse as the oxidation of Fe2+ to Fe3+ (e.g., the H-chain of ferritin), the catalysis of hydroxylation, epoxidation, and desaturation reactions; and the conversion of ribonucleotides into deoxyribonucleotides (e.g., ribonucleotide reductase) (Nordlund and Eklund, 1995; Sazinsky and Lippard, 2006). The common link in all of these dinuclear iron proteins is that they all contain a four helical bundle protein fold, which surrounds a (μ-carboxylato) diiron core with the two iron atoms separated by 0.4 nm or less, and that these iron atoms react with dioxygen (O2). An example of this class of proteins is ribonucleotide reductase; ribonucleotide reductase catalyzes the reduction of the four common ribonucleotides to their corresponding deoxyribonucleotides, which is essential for deoxyribonucleic acid (DNA) synthesis. The structure of the diiron binding site is represented in Figure 36-3 (Nordlund and Eklund, 1995): each Fe2+ ion is octahedrally coordinated by four carboxylate or imidazole groups of the protein. The reaction of the apoenzyme with O2 results in formation of an Fe3+–O–Fe3+ bridge and generation of a tyrosyl free radical in the protein, which plays an essential role in catalysis. Given the many essential functions of iron in oxygen-requiring processes, electron transport, and deoxyribonucleotide synthesis, it is not surprising that efficient mechanisms have developed for iron assimilation and storage. The properties of iron that are so essential for cellular metabolism also make it possible for iron to participate in the generation of highly cytotoxic free radicals, notably the hydroxyl radical. In the aerobic extracellular environment, ferrous ions are readily oxidized to ferric ions, which can rapidly form complexes with hydroxide ions or water to form relatively insoluble polyhydroxides. If these polyhydroxides form in body fluids, the iron becomes unavailable for cellular uptake, and precipitation of these iron aggregates in tissues could have pathological consequences. Consequently, the mechanisms of iron exchange, transport, and storage must maintain an extremely low free iron concentration. Examples of some of the proteins involved in iron transport, storage, and recycling are given in Box 36-2. In addition to the major transport (transferrin and transferrin receptors) and storage (ferritins and hemosiderins) proteins, there are a number of other proteins involved in iron recycling. The major plasma protein involved in the transport of iron is transferrin (Crichton, 2009; Sargent et al., 2005; Wessling-Resnick, 2000). Transferrin, a glycoprotein synthesized principally in the liver, is composed of a single polypeptide chain of about 680 amino acids with two iron-binding sites. The closely related lactoferrin is found in human milk as well as in secretions such as tears and saliva, and is secreted by neutrophils; its principal function is as an iron scavenger preventing the proliferation of invading microorganisms. The transferrins have a characteristic bilobal structure, as shown for the diferric form of human lactoferrin in Figure 36-4, A. The similar amino- and carboxy-terminal lobes of transferrin are organized into two distinct domains, and each lobe contains one iron-binding site. The hexadentate iron binding site (Figure 36-4, C), consists of four protein ligands (an aspartate, a histidine, and two tyrosine residues) plus two oxygen atoms from the synergistically bound carbonate anion (CO32–). Together they form a nearly ideal metal coordination sphere, with tight Fe3+ binding (Kd = 10–19 to 10–20 M–1). Neither Fe3+ nor CO32– is bound significantly in the absence of the other. The multicopper oxidase ceruloplasmin and its enterocytic homolog hephaestin oxidize Fe2+ to Fe3+ before iron is incorporated into apotransferrin (the iron-free form of transferrin). Iron release from transferrin is described later. Binding of iron by transferrin results in striking conformational changes in each of the two lobes (Figure 36-4, B). In the absence of bound Fe3+, the apotransferrin molecule is flexible, with the two domains of each lobe free to swing apart and adopt an open conformation (Figure 36-4, B, left). In the presence of Fe3+ and CO32– they take up a pincerlike closed position around the iron atom, bringing all six ligands involved in iron binding together (Baker et al., 2003). Transferrin receptors are involved in the cellular uptake of iron from the circulation, and they have highest affinity for diferric transferrin. Transferrin receptor 1, is a transmembrane glycoprotein composed of two identical 95-kDa monomers linked by a pair of disulfide bridges. Each monomer consists of 760 amino acid residues organized into an amino-terminal cytoplasmic domain, required for intracellular trafficking of the transferrin–transferrin receptor complex, a short membrane-spanning segment, and a large extracellular domain. The three-dimensional structure of the human transferrin receptor 1-binding extracellular domain has been determined (Lawrence et al., 1999) as has that of the human diferric transferrin–transferrin receptor 1 complex (Cheng et al., 2004), shown in Figure 36-5. Each receptor dimer can bind two transferrin molecules, one to each subunit. The receptor is fundamental to the cellular binding and uptake of iron-bearing transferrin. A second transferrin receptor, transferrin receptor 2, has been identified, which binds transferrin with lower affinity than transferrin receptor 1. It appears to play an important role in the regulation of systemic iron homeostasis. To prevent the unwanted effects of iron-catalyzed free radical generation or iron oxidation and its subsequent precipitation, it is imperative that the cell stores excess iron safely. The iron-storage proteins ferritin and hemosiderin fulfill this cellular “housekeeping” function in all cells; in addition they serve as an iron reservoir in specialized cells (macrophages of the reticuloendothelial system and parenchymal cells of the liver) from which body iron pools can be replenished as iron is used or lost (Liu and Theil, 2005; Crichton, 2009; Arosio and Levi, 2010). The major storage sites of ferritin in normal subjects are liver, spleen, and skeletal muscle. Hemosiderin, which is thought to be the lysosomal degradation product of ferritin, represents a very small fraction of normal body iron stores, and is present mostly in macrophages. It can increase dramatically in iron overload. The iron-free apoferritin molecule is composed of 24 subunits with molecular weights around 20 kDa each and has a total molecular mass of approximately 500 kDa. The apoferritin subunits form a hollow protein shell with an external diameter of 12 to 13 nm, delimiting a cavity of diameter 7 to 8 nm, within which iron can be stored in a nontoxic, water-soluble, yet bioavailable form. In mammalian ferritins, the iron core resembles the mineral known as ferrihydrite. The ferritin subunits (Figure 36-6, A) are roughly cylindrical, 5 nm long and 2.5 nm wide, with approximately 80% of the amino acid sequence present in five α-helices. Ferritin with its 24 subunits has a high degree of symmetry, and Figure 36-6, B and C, show a ferritin molecule viewed down the fourfold and threefold axes of symmetry, respectively (Crichton and Declercq, 2010). The effective storage of iron in human ferritins requires contributions from both subunit types, which play complementary roles in storing, detoxifying, and maintaining Fe3+ in a soluble form. In the first step of iron incorporation, Fe2+ penetrates the apoferritin shell through channels at the surface of the protein and is oxidized at dinuclear iron centers (known as ferroxidase centers) localized in the H subunits. Fe3+ then migrates to nucleation centers within L subunits, in the interior of the protein shell. Once a nucleus of iron core has formed at the nucleation sites, deposition of iron on this inorganic core accomplishes the further incorporation of iron. The maximum storage capacity of each ferritin molecule is approximately 4,500 iron atoms. The mechanism of iron release from ferritin is not at all well understood, although its mobilization appears to require degradation of the ferritin molecule, within both the proteasome and the lysosome (De Domenico et al., 2006). Hemosiderin is a lysosomal storage form of iron, which is found particularly in conditions of iron overload, such as hereditary hemochromatosis and transfusion-dependent hemoglobinopathies such as the thalassemias. The hemosiderins are thought to be the product of lysosomal degradation of ferritin (Crichton, 2009) The iron cores in hemosiderins isolated from iron-loaded animals and humans are ferrihydrite-like, similar to those in ferritin, but are less available to chelation than are those in ferritins (Ward et al., 2000). Total body iron (approximately 40 mg/kg in women and 50 mg/kg in men) is made up of two major and one minor compartment—namely functional, storage, and transport iron—as summarized in Table 36-1. Functional iron is composed predominantly of the iron in hemoglobin of red blood cells and erythroid tissues, iron in myoglobin in the muscle, and iron-containing enzymes present in all cells of the body. The storage iron compartment is intrinsically more variable, accounting for between 0 and 20 mg of iron per kg of body weight. This iron is stored in ferritin, which is located predominantly in parenchymal cells of the liver and skeletal muscle, and in hemosiderin, which is stored mainly in macrophages of the reticuloendothelial system. These storage proteins serve as a repository for dietary iron absorbed in excess of that needed to replace losses from the functional compartment; this provides a mechanism to prevent accumulation of free iron ions, which would be toxic, and to provide an emergency reserve supply from which sudden deficits in the functional compartment may be replenished. Storage iron is usually present in roughly equal amounts in the macrophages of the mononuclear phagocytic cells, in hepatocytes, and in skeletal muscle. A very small amount of ferritin is found in plasma, and the serum ferritin level is used to assess iron stores, but the physiological function of plasma ferritin and its source are not yet clearly defined. TABLE 36-1 Typical Iron Distribution in Adults The major pathways of internal iron exchange between different body compartments are dominated by heme synthesis for erythropoiesis and the turnover of senescent red blood cells (Figure 36-7). Transferrin transports iron between different cellular compartments, with about four fifths of the daily exchange cycling through the erythroid marrow and the mononuclear phagocytic system (i.e., monocytes and macrophages including those in the spleen and lymph nodes). Transferrin-bound iron uptake by cells is mediated by expression of transferrin receptors on their plasma membranes. At physiological pH, the affinity of transferrin for its receptor correlates directly with the degree of iron occupancy. Apotransferrin has negligible affinity for the transferrin receptor at pH 7.4, whereas diferric transferrin has the highest affinity. Iron is delivered to cells via the transferrin-mediated cell cycle, as summarized in the upper left-hand corner of Figure 36-8. The diferric transferrin binds to its receptor and the complex is invaginated into clathrin-coated pits to form vesicles that fuse with endosomes. This delivers the vesicle contents into the interior of the endosome, where the pH is reduced to around 5 to 6 by the action of an ATP-dependent proton pump. Iron release from diferric transferrin is facilitated at acidic pH by protonation of the bound carbonate, thereby weakening the coordination of the iron, and by triggering conformational changes in both transferrin and the transferrin receptor, which stabilize the transferrin molecule in its apo-transferrin conformation. The Fe3+ is then reduced to Fe2+ by members of the six-transmembrane epithelial antigen of the prostate (STEAP) family of metalloreductases (Ohgami et al., 2005, 2006) for transport of Fe2+ out of the late endosome/lysosome into the cytosol via the divalent cation transporter, DMT1. One of the most significant discoveries of the last decade has undoubtedly been the recognition of the key role of ferroportin, the only known cellular iron exporter, in systemic iron homeostasis. Ferroportin, the product of the SLC40A1 gene, was independently identified by three laboratories in 2000 and shown to play a key role in the export of iron from a number of different cell types (McKie et al., 2000; Donovan et al., 2000; Abboud and Haile, 2000). Ferroportin is a transmembrane protein with 10 potential membrane-spanning regions. In polarized epithelial cells, it localizes to the basolateral membrane. It is expressed in many cells that play a critical role in mammalian iron metabolism, including placental syncytiotrophoblasts, duodenal enterocytes, hepatocytes, and reticuloendothelial macrophages. Targeted disruption of the mouse SLC40A1 gene clearly established the unique and nonredundant functions of ferroportin in iron release from cells (Donovan et al., 2005). As illustrated in Figure 36-8 (lower center), ferroportin transports Fe2+ out of the cell, in concert with the ferroxidases (hephaestin in enterocytes and ceruloplasmin in other cell types). The ferroxidases facilitate the extraction of iron from the ferroportin channel and the subsequent loading of Fe3+ onto apotransferrin (De Domenico et al., 2007). Ferroportin expression is regulated by hepcidin, which triggers its degradation. Ferroportin mRNA translation is regulated by the IrRE/IrRP system in macrophages but not in erythroblasts. Since its serendipitous discovery in 2000 (Nicolas et al., 2001; Pigeon et al., 2001), the role of hepcidin as the key regulatory molecule of systemic iron homeostasis has become abundantly clear. It is a member of the defensin family of antimicrobial peptides, although, unlike the defensins whose purpose is to kill bacteria, hepcidin has only weak bactericidal activity. Hepcidin is synthesized by the liver hepatocytes as an 84-residue propeptide, which is cleaved by the protease furin in the Golgi apparatus to yield the mature, bioactive 25-residue peptide that is secreted. Hepcidin, like defensins, forms an amphipathic beta sheet and has four disulfide bonds. Hepcidin is secreted into the bloodstream by the liver, and it acts systemically to downregulate ferroportin (Nemeth et al., 2004). Hepcidin binds to an amino acid sequence in the exterior segment of the transmembrane protein ferroportin, and this triggers ferroportin degradation (De Domenico et al., 2008). Subsequent to hepcidin binding, Janus kinase 2 (Jak 2) binds and phosphorylates ferroportin, resulting in its internalization, ubiquitination, and lysosomal degradation (De Domenico et al., 2009). Upregulation of hepcidin thus decreases iron efflux from the cell as well as its subsequent uptake into transferrin. Hepatic hepcidin expression is regulated at the transcriptional level in response to multiple and in part opposing signals, including systemic iron availability, erythropoietic signals, hypoxia, and inflammatory and stress signals (Zhang and Enns, 2009; Hentze et al., 2010). Iron sensing appears to include mechanisms that respond to transferrin saturation and hepatic iron stores. The inflammatory cytokines interleukin 1 and interleukin 6 are both potent inducers of hepcidin expression. The inflammatory and iron stores regulators appear to operate independently, with integration occurring at the hepcidin gene promoter. Because an increase in hepcidin limits the recycling of heme iron needed to sustain erythropoiesis, upregulation of hepcidin secretion is an important contributor to the anemia of chronic disease. Iron can also be taken up by transferrin-independent pathways. Hemoglobin and heme resulting from intravascular hemolysis are cleared by specific receptor-mediated scavenger systems (see Figure 36-8, upper right). Both the haptoglobin–hemoglobin and the hemopexin–heme complexes are internalized by receptor-mediated endocytosis and degraded. Lipocalin alpha(1)-microglobulin has been proposed to mediate heme uptake. Although the physiological significance of ferritin transport is unknown, some proteins have been suggested to serve a role in ferritin uptake. Ferritin can enter cells via the Scara5 (scavenger receptor class A, member 5) and TIM-2 (T-cell immunoglobulin and mucin domain containing 2) receptors (see Figure 36-8, lower left). Mechanisms of ferritin efflux are unidentified. Iron uptake systems feed into the poorly characterized intracellular transit pool, usually referred to as the labile iron pool (see Figure 36-8), which has been described as “somewhat like the Loch Ness monster—only to disappear from view before its presence, or indeed its nature, can be confirmed” (Crichton, 1984). The labile iron pool represents iron in transit between storage, transport, functional, or recycled forms. Although the exact chemical nature of labile iron remains uncertain, it is thought to represent approximately micromolar concentrations of iron (Breuer et al., 2008). This pool reflects the readily available iron within the cell and is believed to be the key regulator of internal and external iron exchange. The labile iron pool can be utilized (see Figure 36-8) for direct incorporation into iron proteins within the cytosol, or iron can be transported into the mitochondria by the inner mitochondrial membrane protein mitoferrin (see Figure 36-8, right of center). The majority of iron for cellular utilization is channeled through mitochondria-localized pathways that are involved in the insertion of iron into heme and Fe-S cluster prosthetic groups. Mitochondria represent the major subcellular site of iron utilization, so it is not surprising that they play a central role in the control of cellular iron metabolism (Sheftel and Lill, 2009). However, iron management within mitochondria remains poorly understood.
Iron
Biological Functions of Iron
Heme Proteins
Iron-Sulfur Cluster Proteins
Mononuclear Nonheme Iron Proteins
Dinuclear Nonheme Iron Proteins
Proteins of Iron Transport, Storage, and Recycling
Proteins of Iron Transport
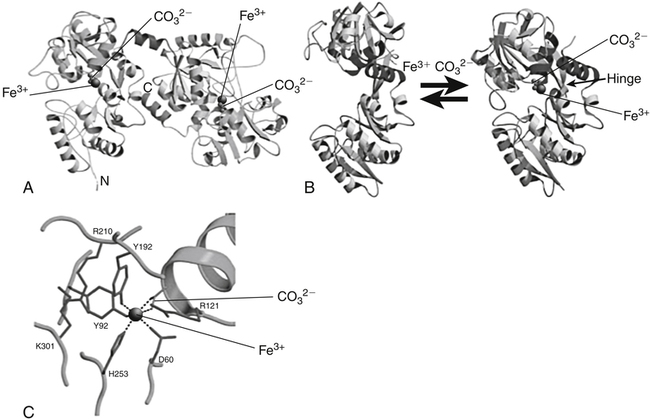
Transferrin
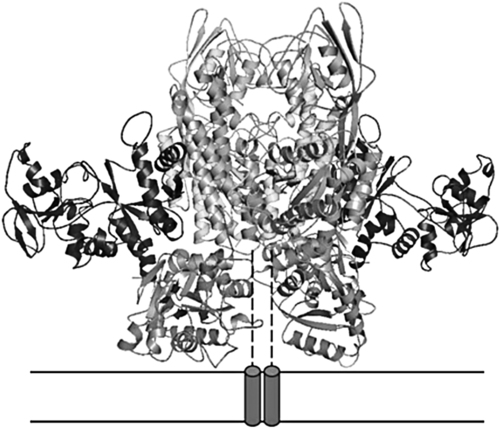
Transferrin Receptors
Proteins of Iron Storage
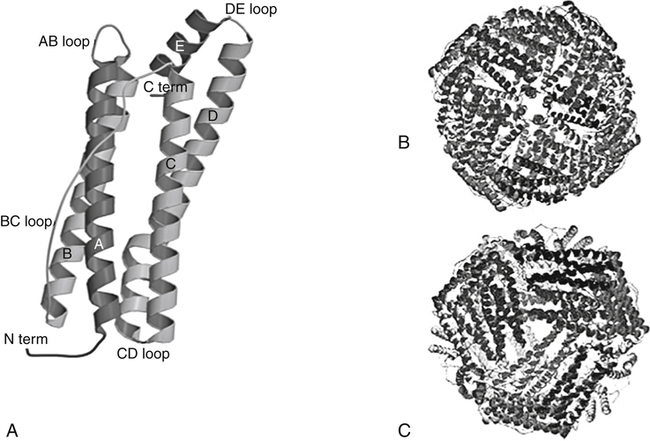
Ferritin and Hemosiderin
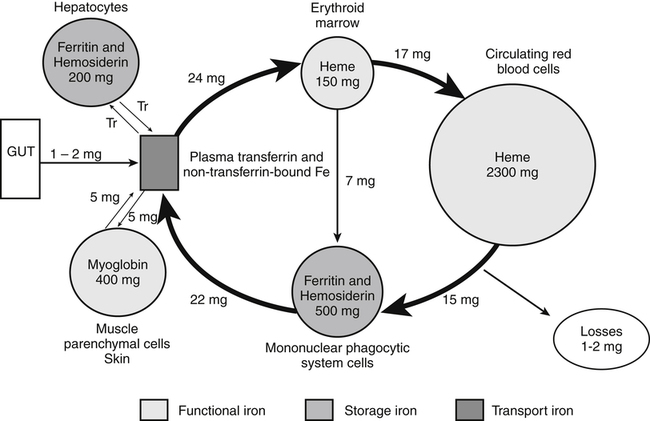
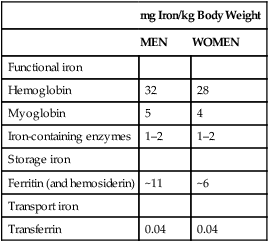
Body Iron Compartments and Daily Iron Exchange
mg Iron/kg Body Weight
MEN
WOMEN
Functional iron
Hemoglobin
32
28
Myoglobin
5
4
Iron-containing enzymes
1–2
1–2
Storage iron
Ferritin (and hemosiderin)
~11
~6
Transport iron
Transferrin
0.04
0.04
Internal Iron Exchange and Cellular Iron Metabolism
Transferrin-dependent Cellular Iron Uptake
Ferroportin, the Cellular Iron Exporter
Hepcidin
Hemoglobin and Heme Uptake and Efflux
Ferritin Transport
Intracellular Iron Trafficking, Utilization, and Storage
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine