Invasive Lobular Carcinoma
SYED A. HODA
The term “lobular carcinoma” became fully established in 1941 with the publication of the classic paper on lobular carcinoma in situ (LCIS) by Foote and Stewart.1 They stated, “When the tumor infiltrates, it is apt to do so in a peculiar fashion which permits one, after some experience, to recognize the high probability of such origin.” An associated desmoplastic stromal reaction, linear arrangement of the carcinoma cells, and their tendency to grow in a circumferential fashion around ducts and lobules (targetoid growth) were “peculiar” diagnostic features emphasized by Foote and Stewart. They also observed that although concurrent LCIS was not found in every case, the histologic pattern was sufficiently distinctive to be considered a specific histologic type of invasive carcinoma that arose from in situ carcinoma of the lobular and terminal duct epithelium.
Invasive lobular carcinoma (ILC), as it was described by Foote and Stewart, is now referred to as classical ILC. In the past 15 years or so, with the recognition that lobular carcinoma is characterized by loss of immunoreactivity for catenins and E-cadherin, classical type of ILC has been distinguished from pleomorphic type of ILC (PILC). Virtually all data about ILC published in the 20th century relate to classical ILC. In this chapter, the abbreviation ILC is used when referring to studies published before and after 2000, unless they refer specifically to PILC.
Less than 5% of invasive mammary carcinomas are composed of a mixture of ductal and lobular histologic features. These mixed carcinomas constituted 3.6% of 4,412 breast carcinomas described in a report by Rakha et al.2 They consist of areas with solid or cohesive growth, sometimes with focal gland-forming elements, typical for invasive ductal carcinoma (IDC) and areas of dyscohesive cells distributed in linear arrays found in ILC. Varying proportions of these components may be present in an individual tumor. The histologic appearance of axillary nodal metastases usually corresponds to the predominant growth pattern of the primary tumors. In situ carcinoma consisting of IDC, ILC, or both is often present. After adjustment for grade, analysis of various markers revealed an expression profile that was intermediate between IDC and ILC but with no significant difference in survival between IDC and ILC.
INCIDENCE
When the diagnosis of ILC is based strictly on the criteria of Foote and Stewart, ILC usually constitutes 5% or less of the carcinomas in most series. Newman3 reviewed 1,396 carcinomas treated over a 17-year period and found that 5% could be classified as ILC, and a review of more than 4,000 carcinomas treated at the Mayo Clinic revealed that 3.2% were ILC.4 An analysis of more than 21,000 breast carcinomas diagnosed in the United States from 1969 to 1971 found 3% classified as infiltrating lobular type.5
A population-based study of women with invasive breast carcinoma diagnosed in the United States between 1987 and 1999 revealed that the incidence of lobular carcinoma increased during this period.6 The increased incidence of ILC was greatest in women aged 50 years or older. On the other hand, the incidence of IDC was relatively constant. Subsequent analysis of data representing 92.1% of the women in the United States between 1999 and 2004 revealed that the age-adjusted incidence of ILC declined by 20.5%, whereas the age-adjusted incidence of IDC and all breast carcinomas were reduced by 14.2% and 11.6%, respectively.7 The authors speculated that the decreased incidence of ILC could be “related to a reduced use of combined hormone replacement therapy.” Chikman et al.8 analyzed 2,175 consecutive patients with breast carcinoma in Israel between 1992 and 2009. During the entire period, 8.6% of the carcinomas were classified as ILC, rising from 4.6% in 1992 to 1994 to 10.9% in 2004 to 2006 and falling to 8.7% in 2007 to 2009. Estrogen-based hormone replacement therapy was used significantly more frequently by women aged 50 to 64 years diagnosed with ILC compared with women diagnosed with IDC.
Allen-Brady et al.9 investigated the relationship between lobular breast carcinoma and the risk of familial breast carcinoma in the Utah Population Database. Among the 22,519 cases of breast carcinoma recorded, 1,453 (6.5%) were classified as lobular type, including in situ and invasive carcinomas. Female first-degree relatives of women with ILC had a significantly increased risk of breast carcinoma, specifically lobular carcinoma, when compared with the risk associated with a history of breast cancer of unspecified type.
CLINICAL PRESENTATION
Age and Sex
ILC occurs throughout virtually the entire age range of breast carcinoma in adult women (28 to 86 years). Most studies have placed the median age at diagnosis between 45 and 56 years.3,4,10,11,12,13,14,15 At the extremes of the age distribution of breast carcinoma,15 ILC is relatively more common among women older than 75 years (11%) than among women 35 years or younger. In an analysis of the age distribution of patients with various types of breast carcinoma, the mean age of women with both ILC and IDC was 57 years.16
Differences have been reported in the age distributions of the classical and variant forms of ILC. Three studies found that patients with classical ILC tended to be younger than those with variant types of ILC.10,12 A fourth study reported that patients with variant tumors had a lower median age at diagnosis (47 years) than those with classical ILC (53 years).14 Buchanan et al.17 found no significant difference in the median age and range of age at diagnosis between patients with ILC (median 61 years; range 34 to 89 years) and those with PILC (median 59 years; range 36 to 86 years).
Human Immunodeficiency Virus
An exceptional instance of ILC occurring in a woman seropositive for human immunodeficiency virus (HIV) has been reported.24 In this case report, tumor progression was observed in the presence of a CD4 count greater than 500 mm-3. Other case reports of breast carcinoma in HIV-positive women have described a variety of histologic tumor types. The evidence for an association between tumor type, clinical course, and immunodeficiency in these patients is inconclusive.25,26,27
Clinical Signs
The presenting symptom in most cases is a mass with illdefined margins, but in some instances, the only evidence of the neoplasm is vague thickening or fine, diffuse nodularity. Large lesions are more likely to cause skin retraction or fixation, but these signs can be encountered with small superficial tumors. Paget disease of the nipple is not ordinarily caused by ILC, but it can develop secondarily when a centrally located tumor extends directly to the epidermis of the nipple.28
Mammography
Classical ILC is not prone to form calcifications, but calcifications may be present coincidentally in benign proliferative lesions such as sclerosing adenosis (SA)29 or when there is florid LCIS (FLCIS) in ducts with necrosis and calcification. Several investigators have reported a much lower frequency of calcifications detected by mammography in ILC than in IDC.30,31,32,33 Kim et al.34 compared the mammographic findings in 27 patients with ILC with 85 patients with IDC. Calcifications were found in the lesion in three ILC cases (11.1%), all of which were associated with a mass. On the other hand, 27 (31.9%) of IDC presented with calcifications, including 14 (16.7%) in which calcifications were the only abnormality. False-negative mammograms were reported in four ILC cases (14.8%) and only one IDC case (1.1%). Brem et al.35 reported negative imaging studies in 21%, 32%, and 17%, respectively, of patients with ILC who had mammography, sonography, and magnetic resonance imaging (MRI). The lowest frequency of false-negative imaging (7%) was obtained with breast-specific gamma imaging (BSGI) that detected six ILC that were not appreciated by mammography and two ILC that were missed by MRI.
In a screening situation, ILC was more likely to be diagnosed clinically during intervals between examinations than by the mammographic screening examination.36 ILC was relatively more frequent among interval carcinomas recorded in a breast cancer-screening program than in the screening-detected group.37 Retrospective analysis revealed that a disproportionate number of interval lesions had been “missed” in the screening process. The only mammographic abnormality in some cases is asymmetrical density or architectural distortion without a distinct mass.34,38
It has been reported that ILC has been associated with a decrease in breast size or volume on mammography, although no change in size was perceived clinically.39 These patients tend to present with thickening rather than a discrete mass on clinical examination, corresponding to diffuse invasive carcinoma in the breast. Kim et al.34 reported decreased breast volume in 22.2% of patients with ILC and 2.3% of those with IDC.
A comparison between mammograms of ILC and other types of carcinoma detected by mammography revealed that lobular carcinomas are more often spiculated and are more often associated with retraction of the nipple or skin.32,40 The most common mammographic manifestations of ILC were asymmetrical, ill-defined, or irregular, spiculated masses.30,32,41 Carcinomas with mixed lobular and duct features tended to have mammographic features intermediate between the groups. The absences of well-defined margins and, in some cases, a tendency to form multiple small nodules throughout the breast are features that may hinder the radiologic detection of ILC and lead to a false-negative diagnosis. Patients with a spiculated mass are less likely to have residual carcinoma when reexcision is performed than are those with ill-defined or asymmetric lesions.41 A minority of ILC are round or oval tumors on mammography.42
Mendelson et al.29 described five mammographic patterns that they found associated with ILC. These included asymmetric density without defined margins, dense spiculated mass, dense breast without a distinct tumor, microcalcifications, and a discrete round mass. Asymmetric, ill-defined density was the most common pattern. It was concluded that ILC did not produce a specific or characteristic mammographic appearance. Mitnick et al.40 did not find a significant
difference in average tumor size among lesions with differing mammographic appearances. The average size of the tumors was 1.2 cm.
difference in average tumor size among lesions with differing mammographic appearances. The average size of the tumors was 1.2 cm.
Sonography
On sonography, Butler et al.43 reported that 60.5% of ILC produced “a heterogeneous hypoechoic mass with angular or ill-defined margins and posterior acoustic shadowing.” The remaining tumors had various other sonographic characteristics, including 12% that were “sonographically invisible.” The sensitivity of sonography for tumors measuring less than 1 cm was 85.7%. Classical ILC tended to produce “focal shadowing without a discrete mass,” whereas tumors with pleomorphic histology were seen as “a shadowing mass.” Tumors of the alveolar, solid, and signet ring cell variety were most often manifested as a “lobulated, well-circumscribed mass.”43 According to Kim et al.,34 posterior acoustic shadowing is seen significantly more frequently with ILC (59.2%) than with IDC. Selinko et al.44 found that the sensitivity of sonography for detecting ILC was 98%, substantially higher than the sensitivity of mammography (65%). The most common sonographic presentation was as a hypoechoic mass, more often with (58%) than without (27%) an acoustic shadow. The authors were also able to use ultrasound to localize axillary lymph nodes (ALN) for fine-needle aspiration (FNA) examination and staging. Albayrak et al.38 observed that sonography detected ILC in 9 of 11 (81.8%) patients with negative mammograms and that it was especially useful in women with mammographically dense breasts. However, sonography can also yield a false-negative result, as reported by Brem et al.35 in 8 of 25 (32%) patients with ILC.
The mammographic estimate of tumor size tends to be less than the grossly measured size in a significant proportion of cases,45 and MRI has been helpful for determining tumor size in these patients.46 Rodenko et al.47 found that MRI was more effective than mammography for determining the extent of the primary ILC in a significant proportion of cases, but the presence of metastatic carcinoma in ALN was not detected in four cases examined. Yeh et al.48 reported that tumor morphology seen on MRI combined with quantitative measurement of gadolinium uptake was effective for detecting ILC in most cases. Ultrasonography has been useful for detecting multifocal and multicentric ILC.49
Magnetic Resonance Imaging
Studies that compared the sensitivity of mammography, sonography, and MRI have usually found MRI to be the most sensitive of the three imaging modalities.34,35,50 The sensitivity of MRI is typically 90% or greater. One of the important advantages of MRI is its high sensitivity for detecting multicentric and multifocal ILC. Mann et al.50 reported that MRI detected secondary foci of ipsilateral carcinoma that were not found by sonography or mammography in 32% of ILC and that occult contralateral carcinoma was detected in 7% of the patients studied only by MRI. Levrini et al.51 described five MRI patterns for ILC, including the presence of multiple small enhancing foci and a dominant lesion surrounded by additional foci. These patterns correspond to multicentric and multifocal distributions. Tumor morphology as seen in the MRI image combined with quantitative measurement of gadolinium uptake was effective for detecting ILC in most cases. ILC is characterized by a slower rate of MRI enhancement than IDC, but peak enhancement is similar for both tumor types.52
Although MRI has not been adopted as a modality for population-based screening on the scale that mammography is used, the foregoing data indicate that it can play an important role in the follow-up of certain high-risk patient groups, including subsets of women with a greater than 20% lifetime risk of developing breast carcinoma such as those with a family history of breast and ovarian carcinoma or a history of mantle irradiation for Hodgkin disease.53 Women with a history of biopsy-proven atypical lobular hyperplasia (ALH) or LCIS are more likely to develop ILC than any other patient group and should have MRI included in a follow-up regimen that also includes mammography and sonography. Because of the increased likelihood that a woman with ILC in one breast may have occult ILC in the opposite breast or develop it later, MRI should also be used in the management of these patients.
PILC cannot be differentiated from the classical type of ILC on imaging studies, although PILC is less often missed. In a study of 22 PILC cases, Jung et al.54 found that one case (5%) was not detected on mammography, and no such case was missed on MRI. On the other hand, 7 (15%) of 46 cases of classical type of ILC were missed on mammography, and 2 (5%) of 40 cases were not detected on MRI. These authors found that 3 (23%) of 13 PILC and 9 (23%) of 40 classical ILC were occult on sonography.
Breast-Specific Gamma Imaging
BSGI has been used for the detection of breast carcinoma; and in one study, it proved to be superior to mammography, sonography, and MRI with a sensitivity of 93%.35 BSGI uses a high-resolution gamma camera to detect differences in the uptake of technetium (Tc) 99m sestamibi between carcinoma cells and normal breast tissue. Brem et al.35 reported that increased tracer uptake was detected in 26 of 28 ILC, including 6 lesions that were not found by mammography and 2 that were missed by MRI. The mean size of ILC detected by BSGI was 20.3 mm (range 2 to 77 mm). Two ILC missed by BSGI measured 5 and 90 mm.
Measuring the Size and Extent of ILC
Determining tumor size is important for preoperative staging of patients with breast carcinoma. Among those with ILC, the mammographic estimate of tumor size tends to be less than the gross measurement of the resected tumor in a significant proportion of cases.45 MRI has proven to be more accurate than either mammography or sonography for measuring the size and extent of ILC when compared with gross measurement.45,46,47,55 Nonetheless, when compared with histologic
examination of the excised specimen, MRI may underestimate the size and extent of ILC in as many as 60% of cases because it is unable to detect microscopic foci of tumor.56
examination of the excised specimen, MRI may underestimate the size and extent of ILC in as many as 60% of cases because it is unable to detect microscopic foci of tumor.56
Microinvasive (T1mic) lobular carcinoma was found in 16 (0.02%) of 75,250 breast carcinomas recorded in one academic medical center over an 18-year period.57 Microinvasion was defined as invasion that measured less than 1 mm. The number of microinvasive foci ranged from 1 to 5 with a mean of 1.5 per case. Eleven cases also had classical LCIS, four had FLCIS, and one had pleomorphic LCIS (PLCIS). When examined at low magnification, the first indication of microinvasive ILC in histologic sections was focal-increased stromal cellularity. Lymph nodes sampled in 13 cases were negative.
Assessment of Lumpectomy Margins
Close or positive margins are a significant issue in patients with ILC who undergo lumpectomy as their primary surgical procedure for ILC in anticipation of breast conservation. Dillon et al.58 reported finding close or positive margins in 38 of 77 (49%) patients with ILC and in 143 of 588 (24%) patients treated for IDC. In this study, mammographic tumor size greater than 1.5 cm and multifocal or multicentric tumor were significant predictors of close or positive margins. A study by Fortunato et al.59 revealed significantly more frequent positive margins at the time of initial tumor excision for ILC (21/171 or 12.3%) than for IDC (71/1,011 or 7%). Close or positive margins were reported in 50 of 101 (50%) patients by Silberfein et al.60 and in 39% of cases studied by Sakr et al.61 Patients whose diagnosis was established preoperatively on the basis of a needle-core biopsy were significantly less likely to have close or positive margins in a lumpectomy specimen, presumably because surgeons aware of the diagnosis of ILC anticipated the possibility of more extensive carcinoma and performed a wider excision.60,62 This conclusion is supported by the data presented by Sakr et al.61 that ascribed the relatively low frequency (39%) of close or positive margins in their series to wider excisions that they described as “full thickness excision” or “oncoplastic surgery” in 73 patients with ILC.
Preoperative MRI reduces the reexcision rate in patients with ILC who undergo breast-conserving surgery.55,60,63 Mann et al.63 found that 27% of women who did not have a preoperative MRI required reexcision because of close or positive margins, whereas reexcision was only done in 9% of cases where preoperative MRI was obtained. Although the data were based on a retrospective review of cases rather than a randomized prospective trial, there was no significant difference in tumor size or the frequency of multifocal tumor between patients who did and did not have preoperative breast MRI. In a prospective study of patients with known ILC who agreed to have preoperative MRI, Lau and Romero64 reported that additional foci of carcinoma were found in 8 of 20 (40%) women with ILC, including 2 with occult contralateral carcinomas. The MRI results led to beneficial changes in surgery for 42% of the patients, and only one patient required reexcision because of a positive margin.
Bilaterality
Patients with ILC are generally considered to have a relatively high frequency of bilateral carcinoma when compared with women who have other types of carcinoma.65 The reported relative risk (RR) of contralateral carcinoma in women with ILC when compared with women with breast carcinoma generally or with women with ductal carcinoma alone ranged from 1.6 (95% confidence interval [CI], 0.7 to 3.6) to 2.0 (95% CI, 0.8 to 8.4) in three studies.65,66,67,68 The wide range of overall bilaterality that has been described (6% to 47%) has been influenced by how the data have been tabulated. Prior and concurrent contralateral carcinomas were present in 6% to 28% of cases.3,4,12,13,69,70,71 The reported incidence of subsequent contralateral carcinoma ranged from 1.069,72 to 2.3873 per 100 women per year or 0.7% per patientyear of follow-up.74 Lee et al.75 estimated the frequency of subsequent contralateral carcinoma to be 10% after 10 years of follow-up. There is some evidence that the frequency of bilaterality is higher in patients with classical ILC than in patients with variant subtypes.73 Follow-up studies have described subsequent contralateral carcinoma in 4% to 14% of patients who had previously been treated for ILC.11,69,71 A lobular component was present in the majority of the synchronous or metachronous contralateral carcinomas, and at least 50% of these were invasive.13,69,70,72 Hislop et al.72 found that patients with stage II infiltrating lobular carcinoma were more likely to develop contralateral carcinoma subsequently than those who have had negative lymph nodes.
In one series, random concurrent contralateral biopsies in 108 patients with ILC revealed ductal carcinoma in situ (DCIS) in 6% and invasive carcinoma in 10% of patients.76 Biopsies performed for clinical indications in an additional 22 cases yielded DCIS in 5% and invasive carcinoma in 32% of patients. The probability of detecting contralateral invasive carcinoma was significantly greater in women who had multicentric ILC in the ipsilateral breast, or if there were ipsilateral lymph node metastases.
Routine biopsy of the contralateral breast is not indicated on the basis of currently available data for patients with ILC.74 When LCIS is excluded, the overall yield of significant findings from this procedure is not greater than for patients with IDC. Contralateral biopsy is appropriate when indicated by imaging or clinical findings and possibly in patients with a strong family history or other evidence of a genetic predisposition to breast carcinoma. The issue of differences in the long-term risk of bilateral breast carcinoma (metachronous as well as synchronous) between patients with IDC and ILC in one breast is unresolved. Data based on patients studied before the use of tamoxifen and other treatments that inhibit the development of contralateral carcinoma may not be directly applicable after the introduction of this and other selective estrogen receptor-modulating drugs.
GROSS PATHOLOGY
Size
The size of ILC ranges from occult, grossly inapparent lesions of microscopic dimensions to tumors that diffusely involve the entire breast. The median and average sizes of measurable tumors are not significantly different from the dimensions of IDC in some studies, but Buchanan et al.17 found that the median size of PILC was significantly greater than that of ILC and IDC (20 vs. 15 vs. 13 mm) in a study of 52 PILC, 356 ILC, and 3,978 IDC.
Typically, ILC forms a firm-to-hard tumor with irregular borders. The edges of the lesion may be more easily appreciated by palpation than by inspection, because the margin may blend imperceptibly with the surrounding parenchyma. Cyst formation, hemorrhage, necrosis, or grossly visible calcifications in the form of “chalky streaks” are generally not present. Most tumors are gray or white, with a scirrhous or fibrous appearance. Cellular variants of ILC are sometimes described as tan.
In some cases, the excised specimen may not be visibly abnormal and only slightly firm to palpation, although substantial involvement by tumor is evident microscopically. This situation was vividly described by Foote and Stewart77:
On some occasions the gross specimens can be quite confusing and misleading. These episodes usually come about at the time of operation when a locally excised specimen of breast tissue is sent in for frozen section diagnosis. Such a specimen may present no distinctly visible lesion and yet contain a palpable area of peculiar induration, the precise limits of which are vague. Such lesions can cause difficulty in frozen section diagnosis. After the diagnosis of cancer is made it is well to be prepared for querulousness from the operating surgeon who understandably would like to be operating for something more finite than indistinct induration.
Another gross manifestation of ILC is the formation of numerous, fine, hard nodules that feel like tiny pebbles or grains of sand in the breast parenchyma. These foci mimic the appearance of SA grossly and microscopically. Indistinct foci of induration or minute nodules may be the only gross evidence of carcinoma in a contralateral breast biopsy specimen when the opposite breast was deemed not to be affected by clinical examination.
T Category
The T category of ILC may be underestimated if it is based solely on the dimension of a grossly apparent tumor because there can be additional grossly inapparent foci of invasive carcinoma. This phenomenon was demonstrated by Moatamed and Apple,78 who compared gross tumor size with the aggregate tumor size of histologically identified ILC in 74 cases. All 26 tumors initially classified as T0 were upstaged (T1, 69%; T2, 19%; and T3, 12%). Among 26 tumors grossly staged as T1, 35% and 15% were restaged as T2 and T3, respectively. Half of the initial T2 tumors were restaged as T3. Overall, 40% to 50% of cases were upstaged when the microscopic extent of carcinoma was taken into consideration. The authors did not correlate gross and microscopic T category with prognostic data such as nodal status or with clinical follow-up. These findings suggest that current tumor (size), regional node (involvement), (distant) metastases (TNM) staging criteria based on the largest single tumor focus may significantly underestimate the primary tumor burden in a substantial number of patients with ILC.
MICROSCOPIC PATHOLOGY
Foote and Stewart77 summarized their definition of the microscopic characteristics of ILC 5 years after they briefly mentioned the lesion in their paper on LCIS. On this second occasion, they emphasized the following:
The infiltrating portions of lobular carcinoma typically reveal thread-like strands of tumor cells rather loosely dispersed throughout a fibrous stroma. After infiltration has occurred there is no tendency for the cells to simulate atypical lobules. Sheet-like growth is distinctly uncharacteristic. … Great cellularity in the primary tumor is unusual but there are occasional cases in which this does occur.
At the cytologic level, the tumor cells were described as “small- or medium-sized,” “rather uniform in their staining properties,” and as exhibiting relatively little “irregularity.” Because of the small size of the carcinoma cells and the extremely dense cellularity sometimes encountered in lymph node metastases, Foote and Stewart cautioned against mistaking such metastases for lymphoma. They noted that the presence of “central mucoid globules” in the tumor cells was a helpful diagnostic feature.1
Classical Form
Foote and Stewart’s77 description of ILC has been widely accepted as defining the classical pattern of this type of carcinoma, and most subsequent clinicopathologic studies adhere closely to this definition. The study of Newman,3 published in 1966, described the first large series of cases. After reviewing histologic sections of 142 tumors that had features of ILC, he determined that 73 could be regarded as “pure” because they exhibited largely or entirely a “singlecell pattern” of growth. He excluded cases in which there was a prominent duct-forming component or the growth pattern was not largely linear.
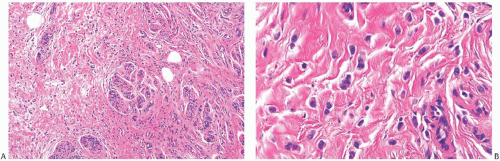
Several growth patterns may be encountered in lesions classified as classical ILC. The tumor cells exhibit a lack of cohesion. The most prominent manifestation of this property in the two-dimensional plane of a histologic section is a tendency to form slender strands of cells arranged in a linear fashion (Figs. 32.1 and 32.2). For the most part, the strands are no more than one or two cells across. Broader bands of cells constitute “trabecular” ILC (Fig. 32.3).
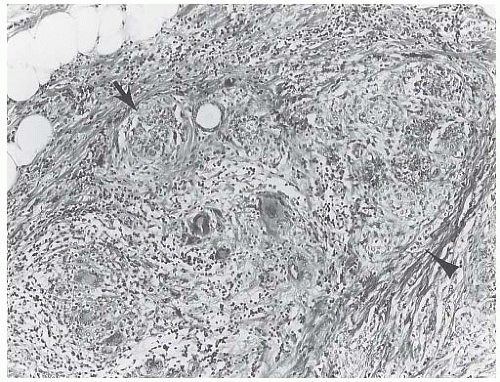
Some tumors feature a growth pattern in which the tumor cells are arranged around ducts and lobules in a concentric fashion (Figs. 32.4 and 32.5). This distribution can have a bull’s-eye or “targetoid” appearance. Inflammatory cells distributed around ducts may mimic the targetoid pattern of ILC, and this phenomenon can present a notable diagnostic problem, especially in a frozen section (FS) or in a needlecore biopsy specimen.79
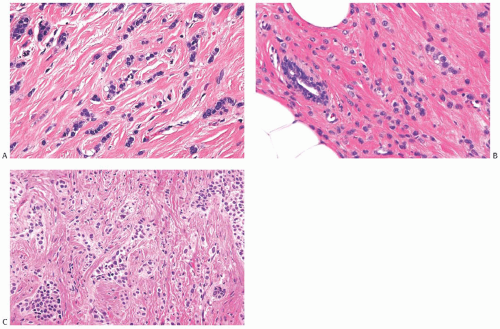 FIG. 32.2. Invasive lobular carcinoma, classical type. A-C: The small carcinoma cells are arranged in slender strands in fibrous tissue. Some clustering of carcinoma cells is evident in (C). |
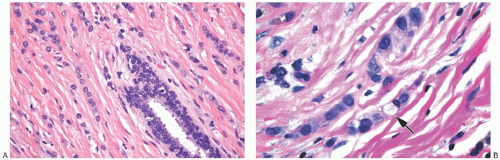
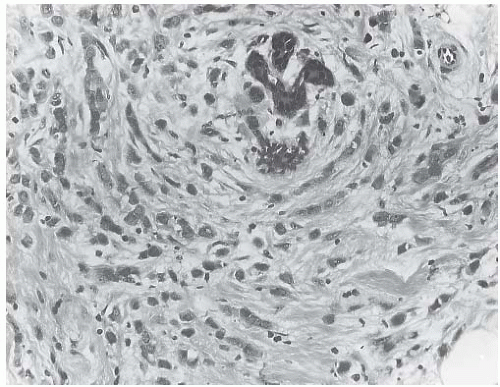
ILC is only rarely accompanied by a lymphocytic reaction (Fig. 32.6). In exceptional cases, an intense lymphoid infiltrate with germinal centers is encountered, and this may suggest a diagnosis of coexistent lymphocytic mastitis80 (Fig. 32.7). Lymphoplasmacytic reaction is found relatively more often in the solid and alveolar variants than in classical ILC. Granulomatous inflammation is very unusual in ILC (Fig. 32.8). The term “lymphoepithelial-like
carcinoma” has been applied to an ILC with a prominent lymphocytic reaction.81 One such tumor was negative for the Epstein-Barr virus.
carcinoma” has been applied to an ILC with a prominent lymphocytic reaction.81 One such tumor was negative for the Epstein-Barr virus.
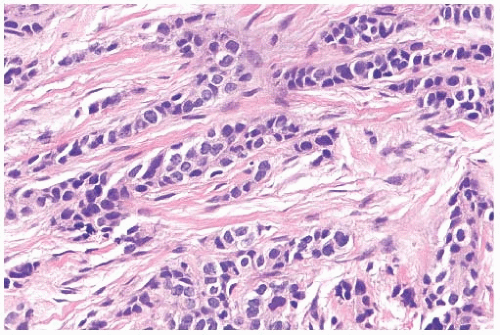 FIG. 32.3. Invasive lobular carcinoma, trabecular type. The classical structure on the top left contrasts with the trabecular pattern elsewhere. |
In a minority of cases, the linear strand-forming pattern is not conspicuous and the tumor cells tend to grow mainly in small dispersed, disorderly foci. This type of invasive growth does not produce a discrete mass and is largely found in patients with little or no gross evidence of invasive carcinoma. The small tumor cells in these invasive foci may be mistaken for lymphocytes or plasma cells in areas of fibrosis or fat when a section is examined at low magnification (Figs. 32.9 and 32.10). The interlobular stroma should be carefully studied for cryptic foci of invasion in biopsies from patients with LCIS (Figs. 32.10, 32.11, to 32.12).
It is quite unusual to encounter an invasive carcinoma in which 100% of the microscopic fields fulfill the foregoing histologic criteria for classical ILC. Many of the tumors composed largely of classical ILC have minor components in which cytologically similar cells exhibit other growth patterns. This situation led Richter et al.4 to limit the diagnosis of ILC to tumors in which at least 70% had a “single-file” growth pattern, and this quantitative criterion has been generally accepted. The diversity of growth patterns is a factor that contributes to problems in reproducible diagnosis reported with ILC.82,83
Variant Forms of Classical ILC
Tumors with the cytologic features of ILC in which there are substantial elements of nonlinear growth have been referred to as “variant” forms of ILC. Areas of classical ILC with a linear pattern are found in most variant forms. “Trabecular” (Fig. 32.3), “alveolar” (Fig. 32.13), and “solid” (Fig. 32.14) variants have been described, extending the diagnosis of ILC to a larger group of tumors. Fechner14 described that six carcinomas in which the confluent growth pattern was characterized as “solid” were composed of cells typically found in classical ILC. The cells were “arranged in irregularly shaped solid nests … sometimes in continuity with a single-file pattern of cytologically identical cells.” Sometimes, these
confluent or solid groups of tumor cells were distributed in circumscribed rounded masses that could be appreciated not only microscopically, but also grossly. No significant differences were observed in age distribution or in tumor size between women with classical and those with the solid variant of ILC. Others found the solid pattern in 9 (41%) of 22 ILC.64 Coexistent LCIS was detected in 10 (45%) of the 22 cases.
confluent or solid groups of tumor cells were distributed in circumscribed rounded masses that could be appreciated not only microscopically, but also grossly. No significant differences were observed in age distribution or in tumor size between women with classical and those with the solid variant of ILC. Others found the solid pattern in 9 (41%) of 22 ILC.64 Coexistent LCIS was detected in 10 (45%) of the 22 cases.
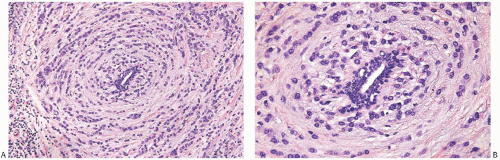 FIG. 32.5. Invasive lobular carcinoma, periductal. A,B: Concentric (“target-like”) infiltration around an inactive duct is shown. |
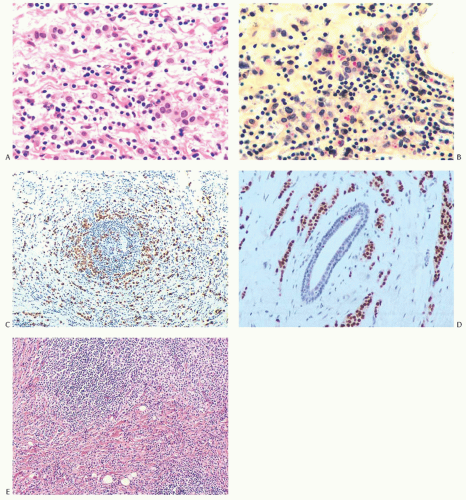 FIG. 32.6. Invasive lobular carcinoma, lymphocytic reaction. A: Carcinoma cells that might be mistaken for histiocytes are distributed among lymphocytes. B: Intracytoplasmic mucin within carcinoma cells is stained red with the mucicarmine stain. C: The carcinoma cells distributed circumferentially in the lymphocytic reaction around an atrophic duct are highlighted by a CK immunostain for AE1/3. D: Nuclear immunoreactivity for ER is shown. E: A nodular lymphocytic infiltrate (above), adjacent to invasive carcinoma. |
Twenty-four examples of “tubulolobular carcinoma” were found among 1,665 tumors in the National Surgical Adjuvant Breast Project (NSABP) series reviewed by Fisher et al.85 These lesions, which constituted 1.4% of the series, were composed of small tubules as well as cords of tumor cells growing in the linear arrangement of classical ILC. Because it had many features intermediate between classical ILC and tubular carcinoma, including a less favorable
prognosis than tubular carcinoma, the authors concluded that tubulolobular carcinoma should be regarded as a separate variant of ILC. Illustrations and a discussion of tubulolobular carcinoma can be found in Chapter 13.
prognosis than tubular carcinoma, the authors concluded that tubulolobular carcinoma should be regarded as a separate variant of ILC. Illustrations and a discussion of tubulolobular carcinoma can be found in Chapter 13.
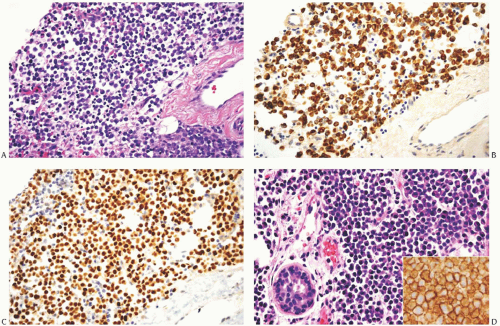 FIG. 32.7. Invasive lobular carcinoma, “plasmacytoid” type. A-C: ILC cells display plasma celltype cytology (A). The invasive carcinoma cells show cytoplasmic reactivity for CK AE1/3 (B) and nuclear immunoreactivity for ER (C). Intramammary solitary plasmacytoma is shown for comparison (D). Inset in (D) reveals reactivity for CD138 that typifies plasma cells. |
The trabecular variant of ILC consists mainly of trabeculae that are one to three cells thick. To be meaningful, the term probably should be restricted to tumors with prominent bands, more than two cells broad. Usually, the trabecular pattern is found in association with other variants, and such tumors are classified as mixed ILC. The “alveolar” pattern is composed of globular aggregates of 20 or more cells, as seen in the plane of a histologic section. The fact that some examples of classical ILC have minor components of alveolar, tubular, trabecular, or solid growth provides evidence to support the classification of neoplasms in which these features are more prominent as variants of ILC. This conclusion is also supported by the fact that these variant forms are negative for E-cadherin.
A major problem in attempts to define and compare the variant forms of ILC has been the rarity of this entire group of tumors and the relatively small numbers of the several
variant types. A series of 230 patients with stage I and stage II ILC included 176 women with classical lesions and 54 (23%) with variant growth patterns.12 Except for a younger age at diagnosis of classical ILC, no clinical differences were found when patients with classical and variant lesions were compared. Women with classical ILC had significantly more frequent ductular extension, and they exhibited a stronger trend to multicentricity that was manifested by greater frequencies of bilaterality as well as gross and microscopic multifocality.
variant types. A series of 230 patients with stage I and stage II ILC included 176 women with classical lesions and 54 (23%) with variant growth patterns.12 Except for a younger age at diagnosis of classical ILC, no clinical differences were found when patients with classical and variant lesions were compared. Women with classical ILC had significantly more frequent ductular extension, and they exhibited a stronger trend to multicentricity that was manifested by greater frequencies of bilaterality as well as gross and microscopic multifocality.
The reported frequency of finding LCIS in association with ILC of the classical type varies considerably. Newman3 found LCIS in 72 (98%) of 73 cases. DiCostanzo et al.12 detected LCIS associated with 65% of 176 examples of classical ILC and 57% of 54 variant tumors. In other smaller series of classical ILC, an LCIS component was found in 31%,86 45%,84 and 87%10 of the cases. In the latter series, proliferative lesions described as ALH and LCIS were grouped together. These authors also reported finding LCIS in association with 56% of 72 variant types of ILC.10
Classical and Pleomorphic Lobular Carcinoma Cells
The cytology of cells that comprise ILC has received considerable attention. All of the cytologic appearances found in LCIS and PLCIS may also be present in ILC. Classical ILC is composed of small, uniform cells with round nuclei and inconspicuous nucleoli. A variable proportion of cells have intracytoplasmic lumina containing sialomucins demonstrable with the mucicarmine and Alcian blue stains87,88 (Fig. 32.15). When the secretion is prominent, the cells have a signet ring configuration (Figs. 32.15 and 32.16). With the aforementioned stains, it is often possible to demonstrate small amounts of secretion in non-signet ring cells. The majority of so-called signet ring cell carcinomas are forms of ILC,12,87,88,89,90 but similar cells are also rarely found in IDC.91,92,93
Some ILC consist entirely or in part of cells with relatively abundant, eosinophilic cytoplasm that are larger than the cells in classical ILC (Fig. 32.17). The nucleus in some examples is hyperchromatic and eccentric with a distinct nucleolus creating a plasmacytoid appearance (Fig. 32.18). These cells have been referred to variously as myoid,12 histiocytoid,94,95,96 and PILC (Figs. 32.18 and 32.19). The hyperchromatic nuclei of PILC cells are typically three to four times larger than a mature lymphocyte. They are often eccentrically positioned in the cell and may be or lobated. One or more nucleoli are sometimes evident. The growth pattern resembles that of ILC, consisting of a diffuse infiltration of dissociated cells that have a linear arrangement and may encircle ducts and lobules. Other histologic evidence that PILC is a variant of lobular carcinoma includes in situ ductal involvement with a “cloverleaf” pattern, the presence of intracytoplasmic mucin and signet ring cells, as well as coexistent classical LCIS and ILC in some cases. This conclusion is supported by a study by Weigelt et al.97 who used gene expression profiling to analyze a series of matched ILC, PILC, and IDC. There was less than a 0.1% difference in gene expression between ILC and PILC, whereas there was a 5.8% difference between ILC and IDC.
When compared with classical ILC, PILC has a higher mitotic rate—a finding that is potentially of prognostic significance.98 In one study, the two types of lobular carcinoma did not differ significantly with respect to intratumoral vascular density.99
Eusebi et al.100 emphasized the presence of apocrine differentiation in PILC and concluded that these patients have an especially aggressive clinical course because 9 of 10 patients in their series developed recurrences. Each of these nine patients had nodal metastases at the time of diagnosis. Apocrine differentiation was also noted by other authors who drew attention to transitions between classical and pleomorphic patterns cell types in the in situ components.96,101 PILC is immunoreactive for gross cystic disease fluid protein 15 (GCDFP-15), a marker of apocrine differentiation.102,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree