Invasive Lobular Carcinoma
Key Facts
Terminology
Invasive lobular carcinomas are characterized by loss of normal cell adhesion and actin cytoskeleton regulation
Responsible for specific morphologic appearance, diffuse pattern of infiltration in the breast and distant sites, and characteristic metastatic pattern
Etiology/Pathogenesis
Approximately 85% lack E-cadherin and 15% lack other cell adhesion proteins
CDH1 germline mutations increase risk of gastric cancer and ILC
Clinical Issues
Most common special type of breast carcinoma: 5-15% of all breast cancers
Majority present as irregular mass
Diffuse pattern of infiltration can make detection difficult by palpation or imaging in around 1/3
Distinct pattern of metastases to serosal surfaces of GI and GYN tracts, leptomeninges, and bone
Prognosis similar to women with carcinomas of no special type matched for grade and stage
Trend toward later recurrence with ILC
Top Differential Diagnoses
Invasive carcinoma with ductal and lobular features
Tubulolobular carcinoma
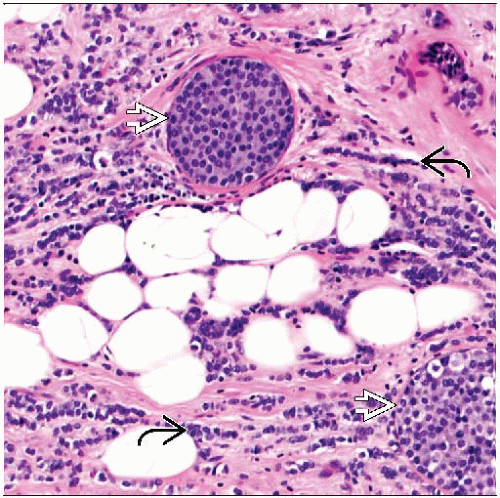
Lymphoid infiltrates and lymphoma
Metastatic melanoma
Myofibroblastoma, epithelioid variant
TERMINOLOGY
Abbreviations
Invasive lobular carcinoma (ILC)
Definitions
Invasive carcinomas characterized by loss of normal cell adhesion and actin cytoskeleton regulation
ILC shows a specific morphologic appearance, typical diffuse pattern of tissue infiltration in breast and distant sites
Distant recurrence will show distinctive metastatic pattern
ETIOLOGY/PATHOGENESIS
Cell Adhesion Protein Expression
Loss of E-cadherin gene (CDH1) expression in approximately 85% of ILC
E-cadherin is a calcium-dependent transmembrane protein
Functional role in intercellular adhesion and cell-polarity
Binds actin cytoskeleton through interactions with p120, α-, β-, and γ-catenin
Loss of E-cadherin affects cellular adhesion, motility, and possibly cell division
Mechanism of loss of E-cadherin expression
1 allele on 16q is inactivated by mutation in ˜ 50-60% of ILC
2nd allele is inactivated by either loss of heterozygosity or promoter hypermethylation
Leads to loss of E-cadherin protein expression as detected by IHC
Expression of E-cadherin but loss of other catenin complex members occurs in approximately 15% of ILC
If E-cadherin is expressed, then 1 or more catenins show abnormal expression
p120 catenin usually shows abnormal cytoplasmic staining
Gene Expression Profiling
ILC of all grades are more similar to each other than to other breast carcinoma types
Majority have luminal A expression profile
Share similar expression patterns related to cell adhesion, cell-to-cell signaling, and actin cytoskeleton signaling
Grade 1 and 2 ILC have distinct gene expression patterns compared to grade 1 and 2 carcinomas of no special type
Germline Mutations of E-cadherin Gene
Hereditary diffuse gastric cancer (HDGC) syndrome is due to germline mutations in E-cadherin gene (CDH1)
Risk of gastric carcinoma is ˜ 40-80% by age ˜ 80
Risk of ILC for females is ˜ 40-50% by age ˜ 80
Gastric signet ring cell carcinoma and ILC are morphologically similar and both lack E-cadherin expression; however, carcinomas have organ-specific gene expression patterns
Some families are detected by predominance of cases of ILC
Majority of women with ILC do not have germline mutations in CDH1
Possibility of germline mutations in other cytoskeletal protein genes is under investigation
Genetic Changes
ILC has fewer chromosomal abnormalities than carcinomas of no special type
3 frequent and consistent changes in all ILC types
Loss at 16q at location of E-cadherin gene (16q22.1)
Gains at 1q and 16p
CLINICAL ISSUES
Epidemiology
Incidence
5-15% of invasive mammary carcinomas
Most common special type of breast carcinoma
Incidence of ILC is rising, primarily among women over 50 years of age
Reasons for rising incidence are uncertain
Unlikely related to increasing use of screening mammography
May be linked to increased use of postmenopausal hormones
Age
More common in older women (> 50 years)
Site
More likely to be multicentric in ipsilateral breast
Contralateral involvement may be slightly higher for ILC than for carcinomas of no special type
However, data is influenced by increased likelihood of bilateral mastectomy or contralateral biopsy
Actual risk for clinical diagnosis of contralateral carcinoma is approximately 0.5-1% per year
Presentation
Poorly defined palpable mass or area of thickening by clinical examination
Irregular mass or architectural distortion by imaging
Treatment
Surgical approaches
Breast conservation is possible
Similar local control and survival if clear margins are achieved
Adjuvant therapy
Majority of ILC are ER positive
Adjuvant endocrine therapy is usually recommended
Neoadjuvant studies have demonstrated that ILC is less responsive to chemotherapy than nonlobular carcinomas
Prognosis
Prognosis similar to women with carcinomas of no special type if matched for grade and stage
Patients with stage I classic ILC may show better recurrence-free survival
Prognosis is related to ILC grade
Better prognosis for classic ILC compared with variant forms
Trend toward late recurrence for ILC
Patients with ILC require long-term clinical follow-up
ILC has distinct pattern of metastatic spread
Serosal and mucosal involvement of GI and GYN tracts and retroperitoneum
Metastatic ILC occasionally seen in GI mucosal biopsies and endometrial curettings
Metastatic ILC to stomach can mimic linitis plastica due to primary gastric carcinoma
IHC panel may be necessary to distinguish metastatic ILC (ER, GCDFP-15, and MUC1 positive) from gastric signet ring cell carcinoma (CDX-2 positive)
Leptomeninges and cerebrospinal fluid involvement
Carcinomatous meningitis is usually due to ILC
Bone
Metastatic ILC can be very difficult to detect in bone marrow due to resemblance to hematopoietic cells
IHC for keratin can be very helpful to determine presence and extent of involvement
Pleural and pulmonary metastases are less common than for other histologic types of carcinomas
IMAGE FINDINGS
Mammographic Findings
Difficult to detect mammographically due to relatively subtle changes in density
Imaging findings due to lack of stromal reaction and diffuse growth pattern in many cases
Metastases may also be difficult to image due to diffuse growth pattern
Typical mammographic findings
Irregular mass
Solid and alveolar variants may present as circumscribed or lobulated masses
Architectural distortion
New focal asymmetry
Calcifications are uncommon
Size may be underestimated by mammogram or ultrasound
MR Findings
Irregular mass with architectural distortion
Foci of septal enhancement
Size may be more accurate by MR examination
ILC can be source of false-negative MR examination
MACROSCOPIC FEATURES
General Features
Macroscopic appearance variable
Majority of ILCs form discrete mass similar to carcinomas of no special type
Some ILC may be difficult to see grossly and are poorly defined
Size
For subtle ill-defined carcinomas, assessment of tumor size for T staging can be difficult
Requires correlation between gross and histologic examination
Number of blocks involved can give estimate of tumor volume
Can be helpful for cases with multiple foci of invasion
MICROSCOPIC PATHOLOGY
Histologic Features
ILC has distinctive cytologic features
Cells are round in shape due to lack of cohesion
Acini, papillae, or other structures requiring cell adhesion are absent
Nuclear grade can vary from grade 1 to grade 3; grade 2 is found in majority of ILC
Cytoplasmic mucin vacuoles may be present
If prominent, cells have signet ring appearance
Cells typically have single vacuole with mucin droplet whereas signet ring cells of GI tract more typically have multiple mucin vacuoles and foamy cytoplasmic appearance
Signet ring cells can also be seen in breast carcinomas of no special type
Distinctive growth pattern
In classical growth pattern, linear arrangements of discohesive cells run in single file between collagen fascicles
Infiltration by bands > 2 cells across has been termed “trabecular” ILC
Single cells may be present
Infiltrating cells may be orientated in circular fashion around normal ducts (targetoid, concentric, or “bull’s eye” appearance)
Skip lesions or patchy growth pattern may be present
Multiple foci of carcinoma may be separated from main lesion by uninvolved breast tissue
Desmoplasia may be minimal or absent
Correlates with absence of discrete mass by imaging or by palpation in some cases
LCIS present in 70-80% of cases
Nuclear grade of LCIS is usually similar to nuclear grade of invasive carcinoma
LCIS is more frequently associated with well- and moderately differentiated ILC
LCIS is less commonly seen in association with variant ILC
Lymph-vascular is very rarely present
Lymph-vascular invasion associated with carcinomas of no special type is likely due to cohesive nests of tumor extending into lymphatic spaces
Because cells of ILC lack cohesion to each other or to vascular wall, likelihood of seeing cells in lymphatics is diminished
Variants of ILC according to growth pattern
Classical: Most common growth pattern
Linear files of single cells (i.e., not alveolar or solid)
Some definitions also require low-grade nuclei; other definitions do not include nuclear grade
Of ILC with classical growth pattern, 80-90% are grade 2, 5-10% grade 1, and 5-10% grade 3
Alveolar
Tumor cells are discohesive but grow in groups of 20 or more separated by fibrovascular septae
Clusters of cells can resemble LCIS
Solid
Tumor cells are present in large sheets with little or no intervening stroma
Cells can be discohesive within mass or show single cell infiltration at edges
Mixed features
ILC showing more than 1 of above patterns
Variants of ILC according to cytologic appearance
Signet ring cell
Signet ring cell morphology is prominent throughout ILC
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree