Invasive Lobular Carcinoma
When the diagnosis is restricted to tumors with the so-called classic histologic appearance, 3% to 4% of carcinomas qualify for a diagnosis of invasive lobular carcinoma (1,2,3). If the classification is broadened to include variant forms, the frequency of invasive lobular carcinoma has reportedly been as high as 10% to 14% of invasive carcinomas (4,5,6). Invasive lobular carcinoma occurs throughout virtually the entire age range of breast carcinoma in adult women (28 to 86 years). Most studies have placed the median age at diagnosis between 45 and 56 years (2,3,5,6,7). Invasive lobular carcinoma is relatively more common among women older than age 75 (11%) than in women age 35 or younger. A population-based study of women with invasive breast carcinoma diagnosed from 1987 through 1999 revealed that the incidence rate of lobular carcinoma increased during this period (8). The increased incidence rate of invasive lobular carcinoma was greatest in women age 50 or older. On the other hand, the incidence rate for invasive ductal carcinoma was relatively constant. In the absence of a systematic pathology review, these data have marginal reliability.
The presenting symptom in almost all patients is a mass, often with ill-defined margins. In some cases, the only evidence of the neoplasm is vague thickening or fine diffuse nodularity of the breast. Invasive lobular carcinoma is not prone to form calcifications, but they may be present coincidentally in benign proliferative lesions, such as sclerosing adenosis (9). A lower frequency of calcifications detected by mammography has been reported in invasive lobular carcinomas than in duct carcinomas (10,11,12). One recently noted exception is invasive lobular carcinoma arising in florid lobular carcinoma in situ with comedonecrosis and calcifications in ducts (see Chapter 21).
The mammographic estimate of tumor size tends to be less than the grossly measured size (13). Rodenko et al. (14) found that magnetic resonance (MR) imaging was more effective than mammography in a significant proportion of cases for determining the extent of a primary invasive lobular carcinoma, but the presence of metastatic carcinoma in axillary lymph nodes was not detected in four cases examined. Yeh et al. (15) reported that MRI tumor morphology combined with quantitative measurement of gadolinium uptake was effective for detecting invasive lobular carcinoma in most cases. However, in the absence of an enhancing mass, this type of carcinoma might not be detectable by MRI.
A comparison between mammograms of invasive lobular and other types of carcinoma revealed that the former were more often spiculated and associated with retraction of the nipple or skin. Carcinomas with mixed lobular and duct features tended to have mammographic features intermediate between the groups. The most common mammographic manifestation of invasive lobular carcinoma is an asymmetric, ill-defined or irregular, spiculated mass (9,10,12,16). In one study, 46% of mammograms from patients who ultimately proved to have invasive lobular carcinoma were initially reported to be negative (11). The absence of well-defined margins, and, in some cases, a tendency to form multiple small nodules throughout the breast, are features that may hinder the radiologic detection of invasive lobular carcinoma and lead to a false negative mammogram interpretation. Patients with a spiculated, invasive lobular carcinoma are less likely to have residual carcinoma when reexcision is performed than are those with ill-defined or asymmetric lesions (16). A minority of invasive lobular carcinomas are mammographically round or oval tumors (17).
Sonography is an important modality for the diagnosis of invasive lobular carcinoma. Selinko et al. (18) reported that the sensitivity of sonography for detecting invasive lobular carcinoma was 98%, substantially higher than the sensitivity of mammography (65%). The most common sonographic presentation was as a hypoechoic mass, more often with (58%) than without (27%) an acoustic shadow. The authors were also able to use ultrasound to localize axillary lymph nodes for a fine needle aspiration (FNA) examination and staging.
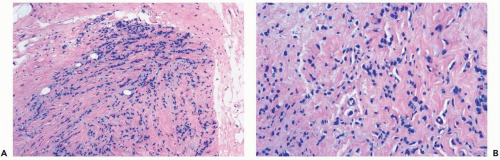
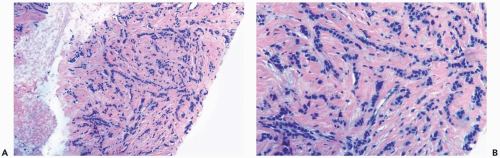 Figure 22.1 Invasive lobular carcinoma, classic type. A, B. The small cells with scant cytoplasm and dark, homogeneous nuclei are arranged in a linear pattern in this needle core biopsy specimen. |
Patients with invasive lobular carcinoma are reported to have a relatively high frequency of bilateral carcinoma when compared with women who have other types of carcinoma (19). Prior and concurrent contralateral carcinomas have been described in 6% to 28% of cases (5,7,20,21). The reported incidence of subsequent contralateral carcinoma ranges from 1.0 (20,22) to 2.38 (23) per 100 women per year. Some evidence reveals that the frequency of bilaterality is higher in patients with classical invasive lobular carcinoma than in patients with variant subtypes (23). A lobular component has been found in the majority of synchronous or metachronous contralateral carcinomas, and at least 50% of these have been invasive (7,20,21,22). In one series, random concurrent contralateral biopsies in 108 patients revealed intraductal carcinoma in 6% and invasive carcinoma in 10% of patients (24). Biopsies performed for clinical indications in an additional 22 cases yielded intraductal carcinoma in 5% and invasive carcinoma in 32%. The probability of detecting contralateral invasive carcinoma was significantly greater in women who had multicentric invasive carcinoma in the ipsilateral breast or who had ipsilateral lymph node metastases.
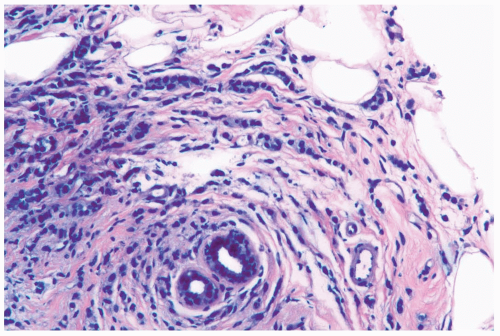 Figure 22.2 Invasive lobular carcinoma, classic type with targetoid growth. The linear infiltrate of carcinoma cells is arranged in circumferential rings around a duct. |
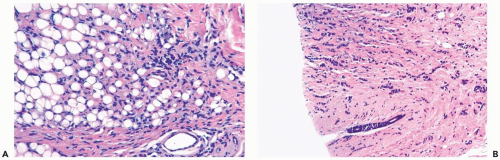
Several growth patterns may be encountered in lesions classified as classical or pure invasive lobular carcinoma. The common denominator is the virtual absence of solid, alveolar, papillary, and gland-forming aggregates of cells. In the two-dimensional plane of a histologic section, the slender strands of cells are arranged in a linear fashion one or two cells across (Fig. 22.1). If the tumor cells are arranged around ducts and lobules in a concentric fashion, the distribution is described as having a targetoid appearance (Fig. 22.2). In a minority of cases, the linear strandforming pattern is not conspicuous, and the tumor cells tend to grow mainly in dispersed, disorderly foci (Fig. 22.3). The small tumor cells in such foci may be mistaken for lymphocytes or plasma cells in areas of fibrosis or in fat when sections are examined at low magnification (Figs. 22.4, 22.5). Invasive lobular carcinoma is only rarely
accompanied by a notable lymphocytic reaction (Fig. 22.6). The term “lymphoepithelioma-like carcinoma” has been applied to an invasive lobular carcinoma with prominent lymphocytic reaction (25). One tumor proved to be negative for Epstein-Barr virus.
accompanied by a notable lymphocytic reaction (Fig. 22.6). The term “lymphoepithelioma-like carcinoma” has been applied to an invasive lobular carcinoma with prominent lymphocytic reaction (25). One tumor proved to be negative for Epstein-Barr virus.
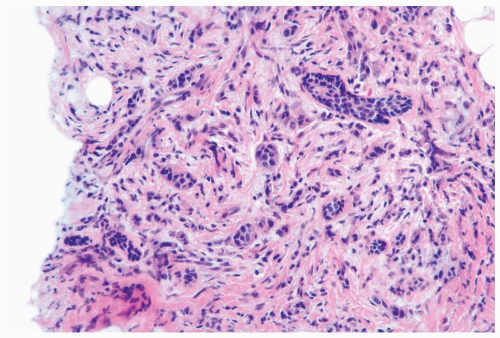 Figure 22.3 Invasive lobular carcinoma, classic type. The linear growth pattern is obscured by stromal reaction around terminal duct and lobular glands in this needle core biopsy specimen. |
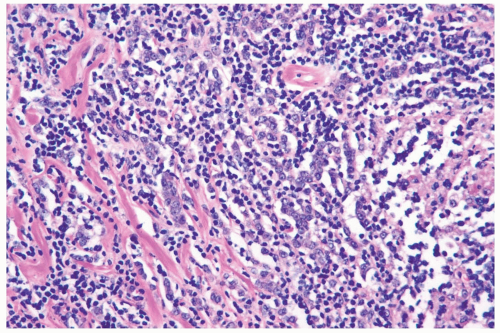 Figure 22.6 Invasive lobular carcinoma with lymphocyte reaction.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|