Potential mechanisms of drug interaction with a receptor. Possible effects resulting from these interactions are diagrammed in the dose-response curves at the right. The traditional agonist (drug A)-receptor binding process results in the dose-response curve denoted “A alone.” B is a pharmacologic antagonist drug that competes with the agonist for binding to the receptor site. The dose-response curve produced by increasing doses of A in the presence of a fixed concentration of B is indicated by the curve “A+B.” Drugs C and D act at different sites on the receptor molecule; they are allosteric activators or inhibitors. Note that allosteric inhibitors do not compete with the agonist drug for binding to the receptor, and they may bind reversibly or irreversibly.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 1-3.)
Inert Binding Sites
Because most drug molecules are much smaller than their receptor molecules (discussed in the text that follows), specific regions of receptor molecules often can be identified that provide the local areas for drug binding. Such areas are termed receptor sites. In addition, drugs bind to other, nonregulatory molecules in the body without producing a discernible effect. Such binding sites are termed inert binding sites. In some compartments of the body (eg, the plasma), inert binding sites play an important role in buffering the concentration of a drug because bound drug does not contribute directly to the concentration gradient that drives diffusion. Albumin and orosomucoid ( 1-acid glycoprotein) are 2 important plasma proteins with significant drug-binding capacity.
1-acid glycoprotein) are 2 important plasma proteins with significant drug-binding capacity.
Pharmacokinetic Principles
To produce useful therapeutic effects, most drugs must be absorbed, distributed, and eliminated. Pharmacokinetic principles make rational dosing possible by quantifying these processes.
The Movement of Drugs in the Body
To reach its receptors and bring about a biologic effect, a drug molecule (eg, a benzodiazepine sedative) must travel from the site of administration (eg, the gastrointestinal tract) to the site of action (eg, the brain).
Permeation
Permeation is the movement of drug molecules into and within the biologic environment. It involves several processes, the most important of which are discussed next.
Aqueous Diffusion
Aqueous diffusion is the movement of molecules through the watery extracellular and intracellular spaces. The membranes of most capillaries have small water-filled pores that permit the aqueous diffusion of molecules up to the size of small proteins between the blood and the extravascular space. This is a passive process governed by Fick’s law (see later discussion). The capillaries in the brain, testes, and some other organs lack aqueous pores, and these tissues are less exposed to some drugs.
Lipid Diffusion
Lipid diffusion is the passive movement of molecules through membranes and other lipid structures. Like aqueous diffusion, this process is governed by Fick’s law (see later discussion).
Transport by Special Carriers
Drugs that do not readily diffuse through membranes may be transported across barriers by mechanisms that carry similar endogenous substances. A very large number of such transporters have been identified, and many of these are important in the movement of drugs or as targets of drug action. Unlike aqueous and lipid diffusion, carrier transport is not governed by Fick’s law and is capacity-limited. Important examples are transporters for ions (eg, Na+/K+ ATPase), for neurotransmitters (eg, transporters for serotonin, norepinephrine), for metabolites (eg, glucose, amino acids), and for anticancer drugs.
Selective inhibitors for these carriers may have clinical value; for example, several antidepressants act by inhibiting the transport of amine neurotransmitters back into the nerve endings from which they have been released. After release, such amine neurotransmitters (dopamine, norepinephrine, and serotonin) and some other transmitters are recycled into nerve endings by transport molecules. Probenecid, which inhibits transport of uric acid, penicillin, and other weak acids in the nephron, is used to increase the excretion of uric acid in gout. The family of P-glycoprotein transport molecules, previously identified in malignant cells as one cause of cancer drug resistance, has been identified in the epithelium of the gastrointestinal tract and in the blood-brain barrier.
Endocytosis, Pinocytosis
Endocytosis occurs through binding of the transported molecule to specialized components (receptors) on cell membranes, with subsequent internalization by infolding of that area of the membrane. The contents of the resulting intracellular vesicle are subsequently released into the cytoplasm of the cell. Endocytosis permits very large or very lipid-insoluble chemicals to enter cells. For example, large molecules such as proteins may cross cell membranes by endocytosis. Smaller, polar substances such as vitamin B12 and iron combine with special proteins (B12 with intrinsic factor and iron with transferrin), and the complexes enter cells by this mechanism. Because the substance to be transported must combine with a membrane receptor, endocytotic transport can be quite selective. Exocytosis is the reverse process, that is, the expulsion of membrane-encapsulated material from cells.
Fick’s Law of Diffusion
Fick’s law predicts the rate of movement of molecules across a barrier; the concentration gradient (C1 – C2) and permeability coefficient for the drug and the area and thickness of the barrier membrane are used to compute the rate as follows:
This relationship quantifies the observation that drug absorption is faster from organs with large surface areas, such as the small intestine, than from organs with smaller absorbing areas (the stomach). Furthermore, drug absorption is faster from organs with thin membrane barriers (eg, the lung) than from those with thick barriers (eg, the skin).
Water and Lipid Solubility of Drugs
Aqueous Diffusion
The aqueous solubility of a drug is often a function of the electrostatic charge (degree of ionization, polarity) of the molecule, because water molecules behave as dipoles and are attracted to charged drug molecules, forming an aqueous shell around them. Conversely, the lipid solubility of a molecule is inversely proportional to its charge.
Lipid Diffusion
Many drugs are weak bases or weak acids. For such molecules, the pH of the medium determines the fraction of molecules charged (ionized) versus uncharged (nonionized). If the pKa of the drug and the pH of the medium are known, the fraction of molecules in the ionized state can be predicted by means of the Henderson-Hasselbalch equation:
“Protonated” means associated with a proton (a hydrogen ion); this form of the equation applies to both acids and bases.
Ionization of Weak Acids and Bases
Weak bases are ionized—and therefore more polar and more water-soluble—when they are protonated. Weak acids are not ionized—and so are less water-soluble—when they are protonated.
The following equations summarize these points:
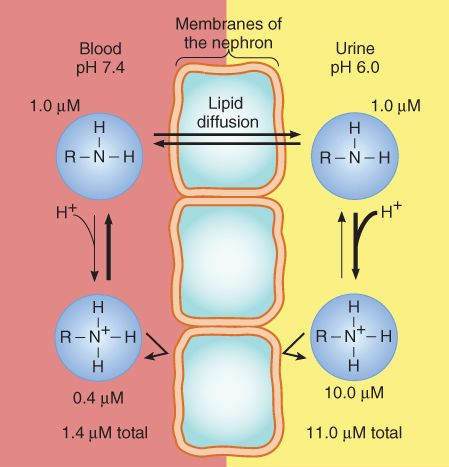
The Henderson-Hasselbalch relationship is clinically important when it is necessary to estimate or alter the partition of drugs between compartments of differing pH. For example, most drugs are freely filtered at the glomerulus, but lipid-soluble drugs can be rapidly reabsorbed from the tubular urine. If a patient takes an overdose of a weak acid drug, for example, aspirin, the excretion of this drug may be accelerated by alkalinizing the urine, for example, by giving bicarbonate. This is because a drug that is a weak acid dissociates to its charged, polar form in alkaline solution, and this form cannot readily diffuse from the renal tubule back into the blood; that is, the drug is trapped in the tubule. Conversely, excretion of a weak base (eg, pyrimethamine, amphetamine) may be accelerated by acidifying the urine, for example, by administering ammonium chloride (Figure 1-2).
FIGURE 1-2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree