A patient may need medicines. In most countries they need a prescription from a medical doctor who thereby shares responsibility with the patient. ‘The’ pharmacist (community, hospital, industrial pharmacist, scientist, teacher or competent authority) is responsible for the supply of the prescribed medicines, being professionally responsible for procurement, medicines design and preparation, storage and dispensing.
The pharmacist needs to assess the doctor’s prescription, for therapy reasons, but within the focus of this book also for availability and, in case of a pharmacy preparation, for safety and quality as well.
The administration route and dosage form strongly influence the design of the medicine and its method of preparation. Chapters 4–14 are written according to the route of administration. These chapters make ample use of examples from preparation in pharmacies, however the design in industrial manufacturing is basically not different. If relevant and possible industrial approaches are touched upon. All design activities need basics independent of the route of administration. These basics are dealt with in the following 7 chapters in a practical pharmaceutical context.
The actual production of medicines is a highly regulated sector of society. The 10 chapters about the different aspects clearly reflect that. Although regulations are omnipresent, the approach of these chapters stems from practice and logic. This also applies to the 3 chapters that cover the control mechanisms of production.
Before the patient can take the prepared or manufactured medicine it has to be stored, procured, and distributed. When the patient has his medicine dispensed, he has to receive labelled or oral instructions, or both, not only for therapeutic reasons but also for keeping and taking the medicine in the right way. Awareness on the impact of medicines on the environment is growing but not integrated yet.
Although many references are in all chapters, the book ends with a list of super references: textbook and hints for postgraduate education.
1.2 Definitions
1.2.1 Types of Pharmacy Preparations
The textbook started its life in the field of pharmacy preparation and although the focus of this edition is on mainstream manufactured products as well, much attention goes to provisions that the hospital or community pharmacist has to offer because not every patient fits the mainstream. It became obvious that official terminology for these peripheral activities is insufficiently discriminating. Therefore a terminology was developed as pictured in Fig. 1.2.
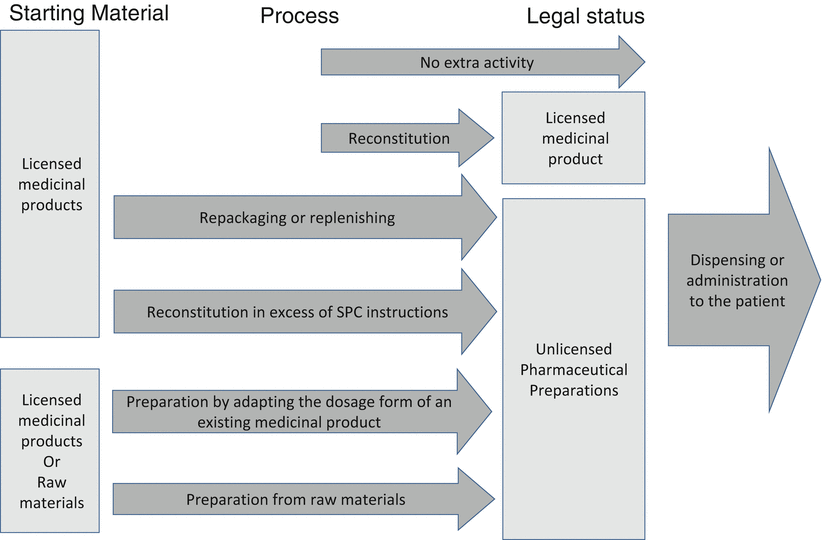

Fig. 1.2
Terminology of preparation activities
The terminology and definition of the activities is as follows:
Reconstitution: manipulation to enable the use or application of a medicinal product with a marketing authorisation in accordance with the instructions given in the summary of product characteristics or the patient information leaflet (Ph. Eur. Pharmaceutical Preparations). Often reconstitution is needed in excess of the instructions of the summary of product characteristics or the patient information leaflet, such as when a longer shelf life is assigned or when a different dilution with an infusion solution takes place. This action is legally considered as preparation. When speaking about the actual work process, that is the handling, it appears not sensible to distinguish between the processes. Therefore this book uses the term ‘reconstitution’ for reconstitution in the strict sense as well as for Reconstitution in excess of the summary of product characteristics or the patient information leaflet. If reconstitution is about parenteral medicines, as is often the case, the term ‘aseptic handling’ may be used in order to distinguish it from aseptic preparation or processing.
Preparation by adapting an existing product: reformulating a licensed product into a different dosage form suitable for the intended use, presented in a suitable and appropriately labelled container (after Ph. Eur. Pharmaceutical Preparations).
Preparation from raw materials: formulating active substances and excipients into a dosage form suitable for the intended use, presented in a suitable and appropriately labelled container (after Ph. Eur. Pharmaceutical Preparations).
1.2.2 Aseptic Preparation, Aseptic Handling and Reconstitution
The reconstitution of parenteral medicines in the strict sense as well in the extended sense (see Sect. 1.2.1) is very frequently performed in hospital pharmacies. The right performance of this process requires extensive precautions on procedures, premises, validation and control. However these differ considerably, due to working with closed systems, from the generally accepted precautions for aseptic processing from raw materials. The use of the term ‘aseptic handling’ therefore was felt justified.
1.3 Terminology
The Editors had to come to an agreement on a number of aspects that required compromise in the usage of descriptive terms and spelling.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


