Figure 5.1 A simple interaction between a liquid and vapor or air
A pure liquid in a container (such as a beaker) with a vapor phase above the bulk liquid.
A simple form of interface is that between a liquid in a beaker and the environment above it (vapor or ‘air’; Figure 5.1). This type of interface is more often referred to as a surface, as the vapor phase many times is not an important consideration, since these are molecules in the air above the liquid, which originate in the bulk. In this example, it can be imagined there will be a lack of symmetry of forces when the two phases meet: the liquid is more dense than the vapor, and is dominated by potential energy of attraction. The forces will be illustrated below. Strong intermolecular interactions keep the liquid in a predominantly condensed state, and potential energy dominates here. Kinetic energy is the dominant force in the vapor phase, where macroscopic actions have their origins in molecular actions.
Between a liquid and its vapor phases is a surface, which is not a true ‘interface’ as the vapor is impure, and the condensed phase is in contact with a vapor. Correctly speaking, the liquid has a surface. The surface of the bulk liquid is in equilibrium with the vapor. If observed from above, the bulk liquid would appear to be a flat, quiescent layer of liquid and have a reasonably definite boundary. One cannot observe the surface of the vapor. Therefore, in this simplest of examples, it must be pointed out that a condensed phase (solid or liquid) in association with a gas will have a surface as opposed to an interface (Figure 5.2). However, many actions that occur at a surface are applicable to interfaces.
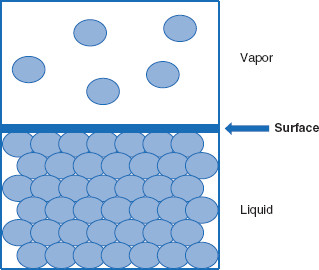
Figure 5.2 Between a condensed phase and a vapor phase is a surface
Between a condensed phase (solid or liquid) and its associated vapor phase is a surface – analogous to, but distinct from, an interface.
A more general term that can be applied to this situation is an interfacial region (Figure 5.3). This term implies a gradual transition between the two phases, whereas the term interface describes a more abrupt delineation. ‘Region’ allows for a changeover between two phases, while the term ‘interface’ describes a demarcation. The term ‘interfacial region’ can be used to recognize the distinction from a true interface, while allowing treatment of surfaces in a similar fashion to interfaces.
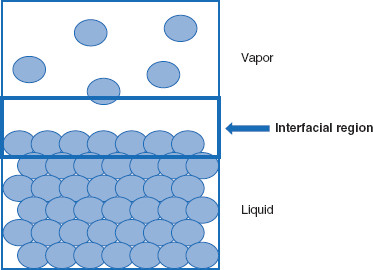
Figure 5.3 Interfacial region between condensed and vapor phases
For a simple condensed phase-vapor phase system, ‘interfacial region’ is a better descriptor than ‘surface’ or ‘interface.’ However, the three terms are often considered synonymous in practice.
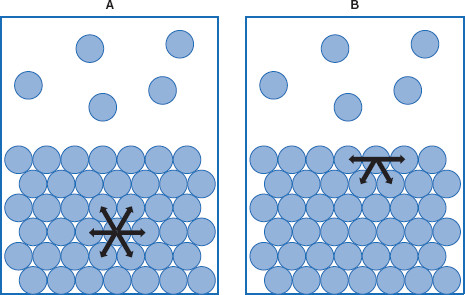
Figure 5.4 Asymmetrical forces exist in the interfacial region
Molecules in bulk (A) are exposed to symmetrical forces from surrounding molecules, while molecules in the interface (B) are pulled into the bulk, minimizing the interfacial area. Both of these should be imagined to be in three dimensions.
Interfacial forces and surface tension
Returning to the asymmetry of forces acting at a surface, it can be illustrated that molecules within the bulk have different molecular interactions than those at the surface. Within the bulk, the interactions tend to cancel each other, resulting in no net force, or symmetrical forces. This is not the case in the interfacial region where forces are not symmetrical (Figure 5.4).
The vapor molecules do exert some pull on the bulk surface molecules, but since the vapor molecules are relatively dilute, their forces on the more densely packed bulk molecules are minimal. The result is that there is a net pull on surface molecules into the bulk. This pull is the cause of the phenomenon observed that liquids reduce their surface area, reducing the number of molecules at the surface as much as possible. The inward pull and reduction of exposed molecules at the surface creates a curved surface (a meniscus) that is often visible. The force that is manifest via the meniscus is called surface tension.
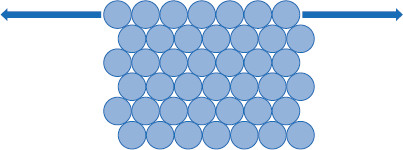
Figure 5.5 Expansion of the liquid interface
In order to oppose unbalanced forces that naturally cause the surface of a liquid to contract and curve, work has to be done against this force.
In order to force the surface of a liquid to increase its surface area, in effect, the edges would need to be ‘pulled’ outward (which, it may be noted, cannot be achieved with a solid; Figure 5.5). To accomplish this pull, work must be done to overcome the thermodynamic tendency of the liquid to minimize the exposed liquid surface.
As the surface area is expanded, molecules from the bulk flow up, into the interface, and so the number of molecules at the surface increases. Work against the pull of the bulk must be done to move the molecules to the surface and enlarge the surface area. Recall that free energy (∆G) is the useful, purposeful energy that can be either obtained from a spontaneous process or that has to be put into a process that is not spontaneous. Since increasing the surface area of the example liquid is not spontaneous, to accomplish this, the Gibbs free energy change (∆G) that is associated with this work is positive (work input is needed). That is, increasing the surface area(s) of a liquid increases the free energy (∆G) of the system as a whole. This work done is against the opposing surface tension (γ) of the liquid. Surface tension is created by the asymmetrical inward pull by the bulk. The amount of work (W) done is proportional to the change in the liquid’s surface area (∆A). This can be represented by the following equation:
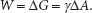
Of interest is the surface tension, γ. So, rearranging the equation provides:
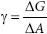
where
W = work (J)
∆G = Gibbs free energy change (J)
γ = proportionality constant = surface or interfacial tension (J/m2)
∆A = change in surface area (m2).
γ is a proportionality constant for the comparison of system free energy and surface area changes. More than that, γ provides a means of gauging what needs to be accomplished in order to cause the changes in G or A to occur, as increasing surface area is a prerequisite to wetting of solids and imparting solubilities to otherwise immiscible materials. It can be noted that γ is a two-dimensional value (i.e., J/m2), rather than three-dimensional (i.e., m3). When using ergs, the units may appear one-dimensional! An erg (dynes × cm) is a force exerted over a distance, meaning γ (dynes/cm) is a force per unit of distance (two-dimensional); ergs/cm2 is the same as dynes/cm and γ is energy (ergs or J/m2) that acts parallel to the surface. In order to increase surface area, work must be done against γ. For water, the work that must be done to overcome surface tension (γ) can be expressed as either 73 ergs/cm2 or 73 dynes/cm (only the units change). Often there arises a need to decrease surface tension (e.g., to improve wetting of a solid by a liquid). In order to decrease the surface tension of a liquid in contact with air, a surface active agent (surfactant) can be employed, as surfactants decrease γ. Surfactants are substances that adsorb at interfaces, decreasing surface tensions between liquids and air, liquids and solids, and between differing liquids. They do so by virtue of being amphiphilic in nature. They decrease contact angles for solids, facilitating wetting and dissolution, and allow dissimilar liquids to mix – not as solutions, but as dispersions.
So far, the condition when a liquid is in contact with its vapor phase has been described, and it has been shown that a surface is present between the two phases. A more complex system involves two immiscible liquids, such as oil and water rather than a liquid and vapor. In this situation, the two liquids will share an interface, rather than a surface. An interface involves more interactions outside of the bulk than occur at a surface (Figure 5.6). In this situation, an interfacial tension rather than a surface tension is present. However, overcoming interfacial tension is analogous to that of surface tension, only the aim is to promote mixing of the two liquids.
When two liquids are in contact, the molecules are distinctly different, but weak (intermolecular) interactions can occur across the interface (i.e., London dispersion forces). So molecules are still pulled into the respective bulk liquids because of lack of symmetry with regard to the forces across the interface of each liquid. This is analogous to interactions between a vapor and a liquid surface, only the pull into the bulk is not as unbalanced as when vapor is the second ‘phase.’ Though the interactions across the liquid–liquid interface are weak, the result is a diminished pull of interfacial molecules into their respective bulk phases. In order to facilitate these interactions, and perhaps induce mixing of otherwise immiscible liquids, as for surface tension, a surfactant can be employed to effect interfacial tension. Surfactants can impact the actions of liquids at interfaces more than at surfaces. Surfactant agents lower surface tension. They also decrease interfacial tension along a liquid surface, between two liquids, but how? Many surfactant molecules are amphiphilic, and so possess some degree of solubility both in oil and water. Recall that amphiphiles are molecules that have both polar and nonpolar regions. The nonpolar region(s) contain hydrocarbon chains. The difference in electronegativity (a means of quantifying the predisposition of an atom to attract electrons) between hydrogen (2.2) and carbon (2.6) is negligible, so there is no large net ‘pull’ in this type of region. The polar region(s) contain groups such as hydroxyls, esters and aldehydes that can be ionic, charged regions. The degree of solubility in each type of liquid depends on the relative proportions of hydrophilic and hydrophobic regions. Ultimately, amphiphiles orient in such a way as to minimize the free energy of the whole system.
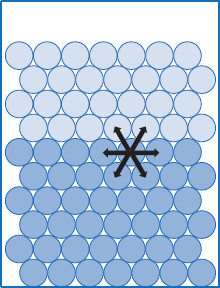
Figure 5.6 Molecular forces in the liquid–liquid interface
Force interactions across a liquid–liquid interface are possible. The higher density of the neighboring liquid phase means molecules are close enough to interact. This results in a diminished pull of interfacial molecules into the bulk of each phase, compared to when only vapor is in contact with a liquid bulk. The two liquids share an interface, rather than a surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


