CHAPTER 27 Integration of Signals
T his chapter summarizes how a variety of well-characterized signal transduction pathways work at the cellular and molecular levels. These examples illustrate diverse mechanisms, but common strategies, for carrying information about changing environmental conditions into cells and for eliciting adaptive responses. Chapters 24 to 26 describe the molecular hardware used in these pathways. Here, the focus is on the flow of information, including examples of branching and converging pathways. For each pathway, the key events are reception of the stimulus, transfer of the stimulus into the cell, amplification of a cytoplasmic signal, modulation of effector systems over time, and adaptation through negative feedback loops. Few signaling pathways operate in isolation; physiological responses usually depend on the integration of pathways.
Detection of Odors by the Olfactory System
Sensory Neurons
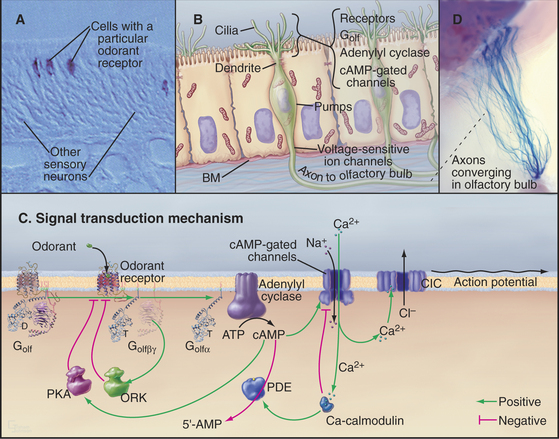
Olfactory sensory neurons located in the nasal epithelium detect specific odorants and respond by sending action potentials to the brain (Fig. 27-1). These neurons have three specialized zones. An apical dendrite extends to the surface of the epithelium and sprouts approximately 12 sensory cilia specialized for responding to particular extracellular odorants. The response depends on high concentrations of four proteins in the ciliary membrane: a single type of odorant receptor, the trimeric G-protein Golf, type III adenylyl cyclase, and cyclic nucleotide–gated ion channels. The cell body contains the nucleus, protein-synthesizing machinery, and plasma membrane pumps and channels that set the resting electrical potential of the plasma membrane. An axon projects from the base of each neuron to secondary neurons in the olfactory bulb at the front of the brain.
Overview of the Pathway
Odorant Receptors
The olfactory system uses a large family of seven-helix receptors to detect a wide range of ligands present at low concentrations in mucus. These receptors were identified by cloning their complementary DNAs (see Fig. 6-8 for cDNAs) from the olfactory epithelium. Genome sequencing established that mice have about 1000 functional odorant receptor genes (about 4% of total genes!), humans have about 350 functional genes, and fish have 100. Odorants are presumed to bind among the transmembrane helices, the most variable part of these proteins. Each sensory neuron typically expresses a single type of odorant receptor (Fig. 27-1A), using negative feedback from the receptor itself to suppress the expression of other types of odorant receptors. The 1000 cells that express each receptor are scattered in zones throughout the olfactory epithelium.
Cyclic Nucleotide–Gated Channels Depolarize the Plasma Membrane and Trigger an Action Potential
The role of cAMP in olfactory signaling was established by experiments on isolated olfactory neurons, in which the effects of odorants, membrane-permeant cyclic nucleotide analogs, and phosphodiesterase inhibitors were explored. Null mutations in mice confirmed the importance of cAMP-gated channels. Note that the role of cAMP in olfaction is distinctly different from its role in most other tissues, where the main target of cAMP is protein kinase A (PKA [see Fig. 25-3]).
Depolarization of the ciliary membrane initiates an action potential (see Fig. 11-6) by activating voltage-gated sodium channels (see Fig. 10-2) in the cell body. The action potential propagates along the axon to a chemical synapse with the second neuron in the olfactory bulb of the brain. The two stages of amplification downstream of the receptor allow a few active receptors to produce an action potential.
Adaptation
Desensitization—the waning of perceived odorant intensity despite its continued presence—results from a combination of central and peripheral processes. Peripheral processes that contribute to adaptation include modulation of each step in the signaling pathway following odorant binding (Fig. 27-1C). At the molecular level, this adaptation is reflected in the transient nature of G-protein activation, the self-limited increase in cAMP, and the limited duration of the membrane depolarization, all of which occur with constant exposure to odorant. Importantly, the sequential nature of many of these feedback circuits implies that they have intrinsic delays and therefore serve not only to alter the magnitude of the response but also to shape its time course.
G-protein-coupled receptors are desensitized by protein kinases that phosphorylate the receptor and by proteins called arrestins that bind phosphorylated receptors (see Fig. 24-3). These modifications inhibit the interaction of activated receptors with G-proteins and provide negative feedback at the first stage of signal amplification. Negative feedback is coupled to receptor stimulation, because the olfactory receptor kinase is brought to the plasma membrane by binding the Gβγ subunits released by receptor-induced G-protein dissociation.
Processing in the Central Nervous System
Mammals discriminate many more odorants than the number of available receptors by combining information from multiple types of receptors in their central nervous systems. While the sensory neurons that express any single odor receptor are broadly distributed across the olfactory epithelium, the axons from this family of like sensory neurons converge on only two to three targets in the olfactory bulb. The target, a glomerulus, is a dense area with synapses between axons of olfactory sensory neurons and dendrites of the second neurons in the pathway. Because each glomerulus receives input only from axons that express the same odor receptor, the molecular specificity established in the olfactory epithelium is preserved. Given approximately 1000 odor receptors in the mouse, each mouse olfactory bulb has approximately 2000 glomeruli (Fig. 27-1D). Of special interest, the odor receptor itself is an important determinant of axon targeting to the glomeruli. Substitution of odor receptors results in the axons selecting new glomerular targets. About 50 secondary neurons receive synaptic input within a glomerulus. Most of these neurons send their axons to higher levels, where they terminate in a combinatorial manner on cortical neurons.
The discrimination of a particular odorant is achieved in two stages: At the first stage, each odorant activates several different receptors, and each receptor can bind a group of related odorants. Therefore, each odorant activates a particular pattern of olfactory sensory neurons and their coupled glomeruli. At the next level, neurons in the cerebral cortex receive information from a combination of glomeruli, leading to eventual discrimination of many different smells at higher levels of the brain. See Box 27-1 for information on our second olfactory system.
BOX 27-1 Sex and the Second Olfactory System
Animals use olfaction to find their mates, identify their offspring, and mark their territories. Some of the odorants that are used for these social interactions are volatile chemicals that stimulate the main olfactory system. A second accessory olfactory system detects other social odorants. Some are volatile chemicals found in urine; others are not volatile, including MHC class II peptide complexes that are shed from the surfaces of cells into the urine and other secretions. Accessory sensory neurons are located in a special part of the epithelium lining nasal cavity called the vomeronasal organ. Each of these neurons expresses one of about 300 seven-helix receptors from a different family than the main odorant receptors. Odorant binding activates a signal transduction pathway distinct from main olfactory neurons, dependent on a Trp channel (see Fig. 10-9) rather than a cyclic nucleotide–gated channel. The axons project to the accessory olfactory bulb in the brain.
Photon Detection by the Vertebrate Retina
Overview of Visual Signal Processing
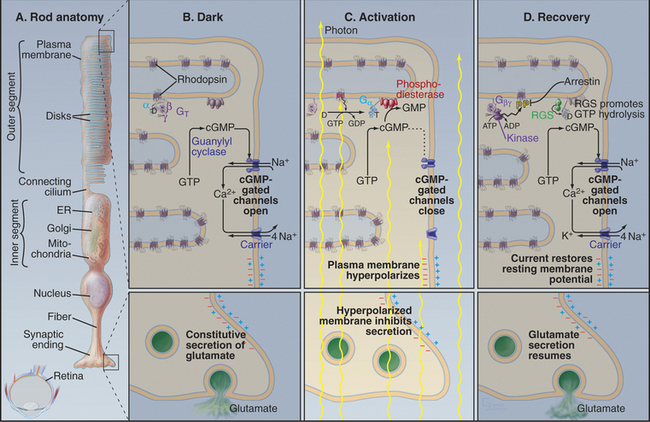
Photons are energetic but unconventional agonists. They are tiny, move very fast, and penetrate most biochemical materials. These properties create a formidable challenge for detecting photons and transducing their properties (intensity and wavelength) into a signal that can be transmitted to the brain. Nevertheless, vertebrate photoreceptor cells capture single photons and convert this energy into a highly amplified electrical response (Fig. 27-2). Phototransduction is the best-understood eukaryotic sensory process because the system is amenable to sophisticated biophysical, biochemical, and physiological analysis. Single-cell organisms use similar mechanisms to respond to light (see Fig. 38-19).
The response of photoreceptor cells depends on the intensity of the light, that is, the flux of photons. Vertebrate retinas detect light with intensities that range over 10 orders of magnitude. Rod photoreceptors (Fig. 27-2A) detect low levels of light from about 0.01 photon per μm2per second (dim stars) to 10 photons per μm2per second but do not discriminate light of different colors. Cone photoreceptors (cones) respond to more intense light, up to about 109photons per μm2per second (full sunlight). Three classes of cones with chromophores that are sensitive to different wavelengths of light allow humans to encode wavelength and color vision to operate (Box 27-2).
Absorption of a photon activates rhodopsin and initiates a signaling cascade (Fig. 27-2) involving a trimeric G-protein and a cGMP phosphodiesterase, both attached to the cytoplasmic face of the disk membrane by covalent lipid groups. Active phosphodiesterase lowers the cytoplasmic concentration of cGMP and closes cGMP-gated channels in the plasma membrane. Closing these channels reduces the release of glutamate at the synapse with the next neuron in the visual circuit. Signals flow through the system as follows:
Feedback loops operate at every level in this signal transduction pathway, turning off the response to a flash of light. The following sections explain how these reactions achieve their spectacular sensitivity in rods.
Rhodopsin
Rhodopsin, the photoreceptor protein of rods, is a seven-helix, G-protein-coupled receptor with a light-absorbing chromophore, 11-cis retinal, covalently attached to lysine 296 through a protonated Schiff base (see Fig. 24-2B). Although 11-cis retinal is bound to a site in the bundle of transmembrane helices similar to sites where ligands bind other seven-helix receptors, this form of rhodopsin is inactive with respect to catalyzing nucleotide exchange on its trimeric G-protein. Thus, rhodopsin is a seven-helix receptor with a covalently attached, but inactive, ligand.
Absorption of light initiates the signal transduction pathway. Picoseconds after the 11-cis retinal chromophore absorbs a photon, the energy isomerizes it to all-trans retinal. This change initiates a cascade of intramolecular reactions that activates rhodopsin by changing its conformation. Metarhodopsin II, the stable active conformation, has rearranged cytoplasmic loops that catalyze nucleotide exchange on transducin, its trimeric G-protein partner (see Fig. 25-9). The signal initiated by absorption of light is amplified by two successive enzymatic reactions and by closing ion channels. Following activation, rhodopsin is inactivated by hydrolysis of the Schiff base linking all-trans retinal to the protein and dissociation of the chromophore. Rhodopsin is regenerated by binding a fresh molecule of 11-cis retinal, derived from vitamin A.
The Positive Arm of the Signal Cascade
As the concentration of cGMP falls, cGMP-gated cation channels in the plasma membrane close. These channels (see Fig. 10-10) are very sensitive to the concentration of cGMP. Binding of four cGMPs opens a channel, whereas the loss of one cGMP closes a channel. Amplification in this pathway is spectacular. Within 1 second after absorption of a single photon, rhodopsin activates 1000 transducins and a similar number of phosphodiesterases, which break down 50,000cGMPs. This change in concentration closes hundreds of cGMP-gated channels, each of which blocks the entry of more than 10,000 cations. Box 27-3 provides more details about the electrical circuit in the rod cell.
Recovery and Adaptation
GTP hydrolysis dissipates the light-activated burst in transducin α-GTP. The low GTPase activity of transducin is activated by association with phosphodiesterase and by an RGS protein (regulator of G-protein signaling; see Fig. 25-8), inactivating transducin in less than 1 second. Humans with mutations that disable the retinal RGS protein cannot adapt to rapid changes in light, so they are blinded for several seconds when they step out of a dark room into full sunlight. Dissociation of transducin α-GDP from phosphodiesterase inhibitory subunits terminates cGMP breakdown.
The reduction in cytoplasmic Ca2+ that accompanies closure of cGMP-gated cation channels stimulates the guanylyl cyclase that rapidly restores the cGMP concentration. This change opens the cation channels and returns the membrane potential to the resting level. See Box 27-4 for information on our second visual system.
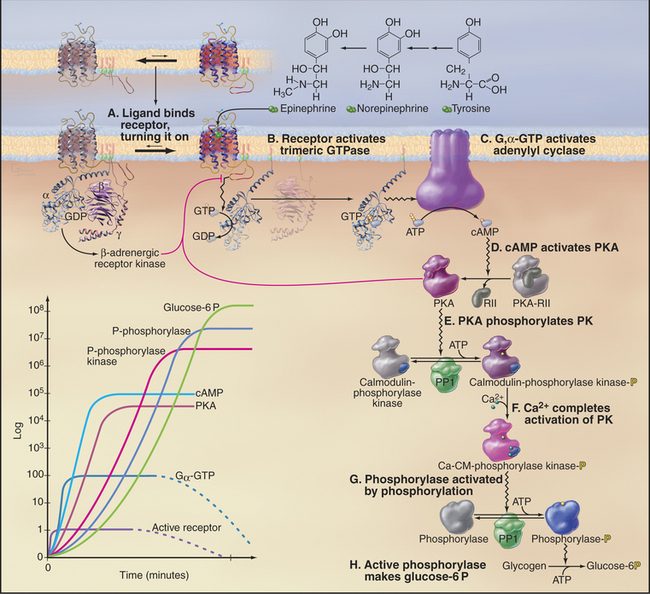
Regulation of Metabolism through the β-adrenergic Receptor
Epinephrine, a catecholamine that is also called adrenaline (Fig. 27-3), is secreted by the neuroendocrine cells of the adrenal gland and other tissues when an animal is startled, is stressed, or otherwise needs to respond vigorously. Norepinephrine, a closely related catecholamine, is secreted by sympathetic neurons, including those that regulate the contractility of the heart. These hormones flow through the blood and stimulate cells of many types throughout the body to heighten their metabolic activity. Skeletal muscle and liver cells respond by breaking down glycogen to glucose to provide energy. Smooth muscle cells of arteries relax to facilitate blood flow. Norepinephrine stimulates heart cells to contract more frequently and with greater force (see Fig. 11-12) and stimulates brown fat cells to dissipate energy as heat (see Fig. 28-6). The variety of physiological responses depends on selective expression of a family of nine adrenergic receptors and their associated signaling hardware in particular differentiated cells (Table 27-1).