Infections in Immunocompromised Patients
KEY CONCEPTS
![]() An immunocompromised host is a patient with defects in host defenses that predispose to infection. Risk factors include neutropenia, immune system defects (from disease or immunosuppressive drug therapy), compromise of natural host defenses, environmental contamination, and changes in normal flora of the host.
An immunocompromised host is a patient with defects in host defenses that predispose to infection. Risk factors include neutropenia, immune system defects (from disease or immunosuppressive drug therapy), compromise of natural host defenses, environmental contamination, and changes in normal flora of the host.
![]() Immunocompromised patients are at high risk for a variety of bacterial, fungal, viral, and protozoal infections. Bacterial infections caused by gram-positive cocci (staphylococci and streptococci) occur most frequently, followed by gram-negative bacterial infections caused by Enterobacteriaceae and Pseudomonas aeruginosa. Fungal infections caused by Candida and Aspergillus, as well as certain viral infections (herpes simplex virus, cytomegalovirus [CMV]), are also important causes of morbidity and mortality.
Immunocompromised patients are at high risk for a variety of bacterial, fungal, viral, and protozoal infections. Bacterial infections caused by gram-positive cocci (staphylococci and streptococci) occur most frequently, followed by gram-negative bacterial infections caused by Enterobacteriaceae and Pseudomonas aeruginosa. Fungal infections caused by Candida and Aspergillus, as well as certain viral infections (herpes simplex virus, cytomegalovirus [CMV]), are also important causes of morbidity and mortality.
![]() Risk of infection in neutropenic patients is associated with both the severity and duration of neutropenia. Patients with severe neutropenia (absolute neutrophil count < 500 cells/mm3 [<0.5 × 109/L]) for greater than 7 to 10 days are considered to be at high risk of infection.
Risk of infection in neutropenic patients is associated with both the severity and duration of neutropenia. Patients with severe neutropenia (absolute neutrophil count < 500 cells/mm3 [<0.5 × 109/L]) for greater than 7 to 10 days are considered to be at high risk of infection.
![]() Fever (single oral temperature of ≥38.3°C [101°F], or a temperature of ≥38°C [100.4°F] for ≥1 hour) is the most important clinical finding in neutropenic patients and is usually the stimulus for further diagnostic workup and initiation of antimicrobial treatment. Infection should be considered as the cause of fever until proven otherwise. Usual signs and symptoms of infection may be altered or absent in neutropenic patients. Appropriate empiric broad-spectrum antimicrobial therapy must be rapidly instituted to prevent excessive morbidity and mortality.
Fever (single oral temperature of ≥38.3°C [101°F], or a temperature of ≥38°C [100.4°F] for ≥1 hour) is the most important clinical finding in neutropenic patients and is usually the stimulus for further diagnostic workup and initiation of antimicrobial treatment. Infection should be considered as the cause of fever until proven otherwise. Usual signs and symptoms of infection may be altered or absent in neutropenic patients. Appropriate empiric broad-spectrum antimicrobial therapy must be rapidly instituted to prevent excessive morbidity and mortality.
![]() Empiric antimicrobial regimens for neutropenic infections should take into account patients’ individual risk factors, as well as institutional infection and susceptibility patterns. The significant morbidity and mortality associated with gram-negative infections require that initial empiric regimens for treatment of febrile neutropenia have good activity against P. aeruginosa and Enterobacteriaceae. Inpatient parenteral regimens most commonly recommended for initial treatment include monotherapy with an antipseudomonal β-lactam, or a combination regimen consisting of an antipseudomonal β-lactam, plus an aminoglycoside. Low-risk patients may be successfully treated with oral antibiotics (ciprofloxacin plus amoxicillin/clavulanate), with the treatment setting determined by the patient’s clinical status.
Empiric antimicrobial regimens for neutropenic infections should take into account patients’ individual risk factors, as well as institutional infection and susceptibility patterns. The significant morbidity and mortality associated with gram-negative infections require that initial empiric regimens for treatment of febrile neutropenia have good activity against P. aeruginosa and Enterobacteriaceae. Inpatient parenteral regimens most commonly recommended for initial treatment include monotherapy with an antipseudomonal β-lactam, or a combination regimen consisting of an antipseudomonal β-lactam, plus an aminoglycoside. Low-risk patients may be successfully treated with oral antibiotics (ciprofloxacin plus amoxicillin/clavulanate), with the treatment setting determined by the patient’s clinical status.
![]() Neutropenic patients who remain febrile after 3 to 5 days of initial antimicrobial therapy should be reevaluated to determine whether treatment modifications are necessary. Common regimen modifications include addition of vancomycin (if not already administered) and antifungal therapy (amphotericin B or fluconazole). Therapy should be directed at causative organisms, if identified, but broad-spectrum regimens should be maintained during neutropenia.
Neutropenic patients who remain febrile after 3 to 5 days of initial antimicrobial therapy should be reevaluated to determine whether treatment modifications are necessary. Common regimen modifications include addition of vancomycin (if not already administered) and antifungal therapy (amphotericin B or fluconazole). Therapy should be directed at causative organisms, if identified, but broad-spectrum regimens should be maintained during neutropenia.
![]() The optimal duration of therapy for febrile neutropenia is controversial. The decision to discontinue antimicrobials is based on resolution of neutropenia, defervescence, culture results, and clinical stability of the patient.
The optimal duration of therapy for febrile neutropenia is controversial. The decision to discontinue antimicrobials is based on resolution of neutropenia, defervescence, culture results, and clinical stability of the patient.
![]() Prophylactic antimicrobials are administered to cancer patients expected to experience prolonged neutropenia, as well as to both hematopoietic stem cell and solid-organ transplant recipients. Prophylactic regimens may include antibacterial, antifungal, antiviral, or antiprotozoal agents, or a combination of these, selected according to risk of infection with specific pathogens. Optimal prophylactic regimens should take into account individual patient risk for infection and institutional infection and susceptibility patterns.
Prophylactic antimicrobials are administered to cancer patients expected to experience prolonged neutropenia, as well as to both hematopoietic stem cell and solid-organ transplant recipients. Prophylactic regimens may include antibacterial, antifungal, antiviral, or antiprotozoal agents, or a combination of these, selected according to risk of infection with specific pathogens. Optimal prophylactic regimens should take into account individual patient risk for infection and institutional infection and susceptibility patterns.
![]() Patients undergoing hematopoietic stem cell transplantation are at an extremely high risk of infection because of prolonged neutropenia following intensive chemotherapy ± irradiation, while solid-organ transplant recipients are at high risk because of prolonged administration of immunosuppressive drugs. Fungal (Aspergillus) and viral (CMV) infections are particularly troublesome in these populations, and prophylactic regimens directed against these pathogens are commonly used. When documented, these infections must be treated aggressively in order to optimize patient outcomes. Nevertheless, mortality rates are often high despite appropriate and aggressive antimicrobial therapy.
Patients undergoing hematopoietic stem cell transplantation are at an extremely high risk of infection because of prolonged neutropenia following intensive chemotherapy ± irradiation, while solid-organ transplant recipients are at high risk because of prolonged administration of immunosuppressive drugs. Fungal (Aspergillus) and viral (CMV) infections are particularly troublesome in these populations, and prophylactic regimens directed against these pathogens are commonly used. When documented, these infections must be treated aggressively in order to optimize patient outcomes. Nevertheless, mortality rates are often high despite appropriate and aggressive antimicrobial therapy.
![]() Immunocompromised patients must be continuously assessed for evidence of infection and response to antimicrobial therapy. Because a large number of antimicrobials may potentially be used, the occurrence of drug-related adverse effects must also be carefully assessed. Efforts should be directed at designing cost-effective treatment strategies that promote optimal patient outcomes.
Immunocompromised patients must be continuously assessed for evidence of infection and response to antimicrobial therapy. Because a large number of antimicrobials may potentially be used, the occurrence of drug-related adverse effects must also be carefully assessed. Efforts should be directed at designing cost-effective treatment strategies that promote optimal patient outcomes.
An immunocompromised host is a patient with intrinsic or acquired defects in host immune defenses that predispose to infection. Advances in modern medicine have created more immunocompromised hosts than ever before. Historically, many of these patients died of their underlying diseases. Dramatic improvements in survival have been achieved by more aggressive therapy of underlying diseases and improved supportive care. However, because such aggressive therapy often renders patients profoundly immunosuppressed for long periods, opportunistic infections remain important causes of morbidity and mortality. This chapter focuses on risk factors for infection, common pathogens and infection sites, and prevention and management of suspected or documented infections in cancer patients (including hematopoietic stem cell transplantation [HSCT] patients) and solid-organ transplant (SOT) recipients. Chapter 103 discusses infectious complications associated with human immunodeficiency virus (HIV) infection.
RISK FACTORS FOR INFECTION/EPIDEMIOLOGY
Many factors influence the degree of immunosuppression and also influence the epidemiology of the associated infections.
Neutropenia
![]()
![]()
![]() Neutropenia is defined as an abnormally reduced number of neutrophils circulating in peripheral blood. Although exact definitions of neutropenia can vary, an absolute neutrophil count (ANC) of less than 1,000 cells/mm3 (1.0 ×109/L) indicates a reduction sufficient to predispose patients to infection.1 ANC is the sum of the absolute numbers of both mature neutrophils (polymorphonuclear cells [PMNs], also called polys or segs) and immature neutrophils (bands). The absolute number of PMNs and bands is determined by dividing the total percentage of these cells (obtained from the white blood cell [WBC] differential) by 100 and then multiplying the quotient obtained by the total number of WBCs (expressed in cells/mm3).
Neutropenia is defined as an abnormally reduced number of neutrophils circulating in peripheral blood. Although exact definitions of neutropenia can vary, an absolute neutrophil count (ANC) of less than 1,000 cells/mm3 (1.0 ×109/L) indicates a reduction sufficient to predispose patients to infection.1 ANC is the sum of the absolute numbers of both mature neutrophils (polymorphonuclear cells [PMNs], also called polys or segs) and immature neutrophils (bands). The absolute number of PMNs and bands is determined by dividing the total percentage of these cells (obtained from the white blood cell [WBC] differential) by 100 and then multiplying the quotient obtained by the total number of WBCs (expressed in cells/mm3).
The degree or severity of neutropenia, rate of neutrophil decline, and duration of neutropenia are important risk factors for infection.1–5 All neutropenic patients are considered to be at risk for infection, but those with ANC less than 500 cells/mm3 (0.5 × 109/L) are at greater risk than those with ANCs of 500 to 1,000 cells/mm3 (0.5 × 109 to 1.0 × 109/L). Most treatment guidelines use ANC less than 500 cells/mm3 (0.5 × 109/L) as the critical value in making therapeutic decisions regarding the management of suspected or documented infections.1–5 Risk of infection and death are greatest among patients with less than 100 neutrophils/mm3 (0.1 × 109/L) (“profound neutropenia”).1,2,5 In patients with chemotherapy-induced neutropenia, the risk of infection is also increased according to both the rapidity of ANC decline and duration of neutropenia. Patients with severe neutropenia of more than 7 to 10 days’ duration are considered to be at especially high risk for serious infections.3,5 The duration of chemotherapy-induced neutropenia varies considerably among subsets of cancer patients according to the specific chemotherapeutic agents used and the intensity of treatment. Patients undergoing HSCT may have no detectable granulocytes in peripheral blood for up to 3 to 4 weeks and are at particular risk for severe infections with a variety of pathogens.6
Bacteria and fungi commonly cause infections in neutropenic patients. Gram-positive cocci (Staphylococcus aureus, Staphylococcus epidermidis, and other coagulase-negative staphylococci, streptococci, and enterococci) have emerged as the most common cause of acute bacterial infections among neutropenic patients. Gram-negative bacilli (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) traditionally were the most common causes of bacterial infection and remain frequent pathogens.4,7–9 Although now not as common as gram-positive bacteria, the incidence of gram-negative infections may again be increasing.3,8 Gram-negative infections are associated with significant morbidity and mortality, in large part due to increasing antibiotic resistance.7–9 Patients who are neutropenic for extended periods and who receive broad-spectrum antibiotics are at high risk for fungal infections, usually due to Candida or Aspergillus spp.2,3,10,11 Viral infections, although not as common as bacterial and fungal infections, also may cause severe infection in neutropenic patients.2,3,5,6 Successful treatment of infections in neutropenic patients depends on resolution of neutropenia.1–3,5
Although not readily quantifiable, abnormalities may exist in granulocyte function as well as in cell numbers. Defects in phagocyte function may be caused by underlying disease (e.g., leukemia) or its treatment (e.g., corticosteroids, antineoplastic agents, and radiation).3,12
Immune System Defects
In addition to neutropenia, defects in T-lymphocyte and macrophage function (cell-mediated immunity), B-cell function (humoral immunity), or both predispose patients to infection. Cellular immune dysfunction is the result of underlying disease or immunosuppressive drug therapy; these defects result in a reduced ability of the host to defend against intracellular pathogens. Patients with Hodgkin’s disease and transplant patients receiving a wide variety of immunosuppressive drugs, such as cyclosporine, tacrolimus, sirolimus, mycophenolate, corticosteroids, azathioprine, and antineoplastic agents, are at risk for a variety of bacterial, fungal, viral, and protozoal infections (Table 100-1). Although some of these pathogens are associated with asymptomatic or mild disease in normal hosts, they may cause disseminated, life-threatening infections in immunocompromised hosts.
TABLE 100-1 Risk Factors and Common Pathogens in Immunocompromised Patients
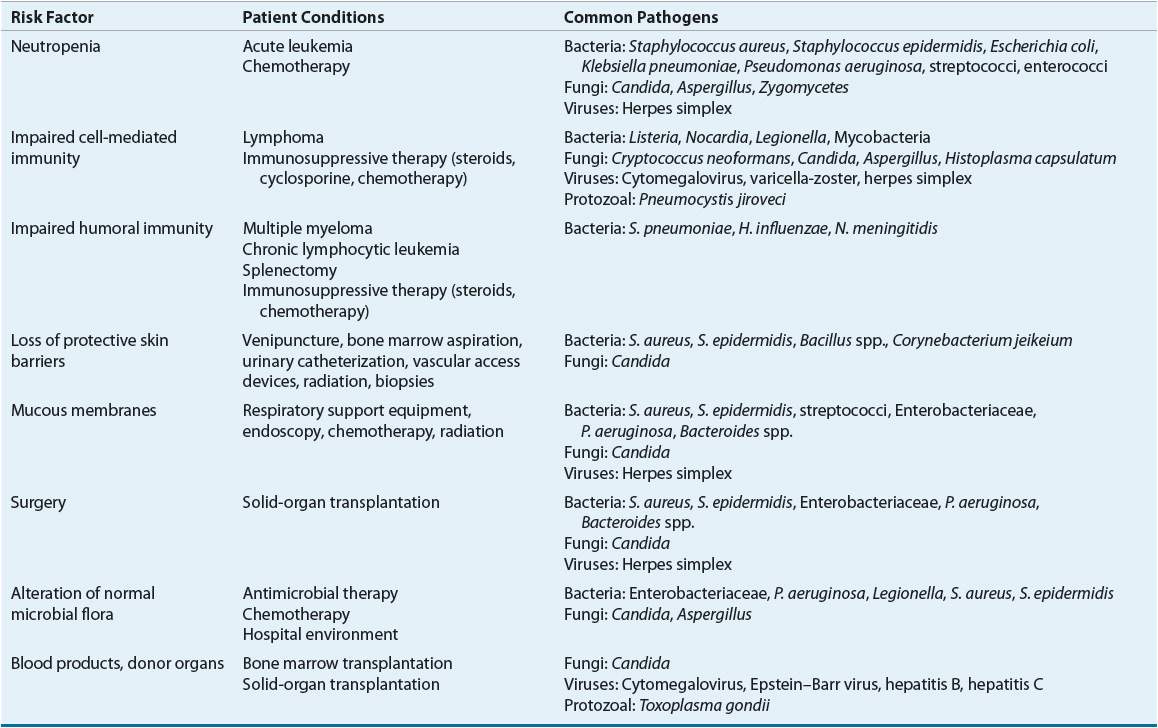
Underlying disease also frequently causes defects in humoral immune function. Patients with multiple myeloma and chronic lymphocytic leukemia have progressive hypogammaglobulinemia that results in defective humoral immunity. Splenectomy performed as a part of the staging process for Hodgkin’s disease places patients at risk for infectious complications. Disease states with humoral immune dysfunction predispose the patient to serious, life-threatening infection with encapsulated organisms such as Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis.
Destruction of Protective Barriers
Loss of protective barriers is a major factor predisposing immunocompromised patients to infection. Damage to skin and mucous membranes by surgery, venipuncture, IV and urinary catheters, radiation, and chemotherapy disrupts natural host defense systems, leaving patients at high risk for infection. Chemotherapy-induced mucositis may erode mucous membranes of the oropharynx and GI tract and establish a portal for subsequent infection by bacteria, herpes simplex virus (HSV), and Candida.3,5,6 Medical and surgical procedures, such as transplant surgery, indwelling IV catheter placement, bone marrow aspiration, biopsies, and endoscopy, further damage the integument and predispose patients to infection. Infections resulting from disruption of protective barriers usually are a result of skin flora, such as S. aureus, S. epidermidis, and various streptococci.1,3,5,12
Environmental Contamination/Alteration of Microbial Flora
Infections in immunocompromised patients are caused by organisms either colonizing the host or acquired from the environment. Microorganisms may be transferred easily from patient to patient on the hands of hospital personnel unless strict infection control guidelines are followed. Contaminated equipment, such as nebulizers or ventilators, and contaminated water supplies have been responsible for outbreaks of P. aeruginosa and Legionella pneumophila infections, respectively. Foods, such as fruits and green leafy vegetables, which often are colonized with gram-negative bacteria and fungi, are sources of microbial contamination in immunocompromised hosts.3,6,13
Most infections in cancer patients are caused by organisms colonizing body sites, such as the skin, oropharynx, and GI tract.1,3,5,6,13 Approximately 80% of infecting bacterial pathogens are from the patient’s own endogenous flora.1,3 The GI tract is the most common site from which infections in immunocompromised hosts originate. Periodontitis, pharyngitis, esophagitis, colitis, perirectal cellulitis, and bacteremias are caused predominantly by normal flora of the gut; bloodstream infections are thought to arise from microbial translocation across injured GI mucosa.1,5,6,13 Normal flora may be significantly disrupted and altered; oropharyngeal flora rapidly change to primarily gram-negative bacilli in hospitalized patients. Many cancer patients may already be colonized with gram-negative bacilli on admission as a result of frequent prior hospitalizations and clinic visits. In hospitalized cancer patients, however, up to 50% of infections are caused by colonizing organisms acquired after admission.1,3
Although hospitalization and severity of illness are important risk factors for colonization by gram-negative bacilli, administration of broad-spectrum antimicrobial agents has the greatest impact on flora of immunocompromised hosts. Use of these agents disrupts GI tract flora and predisposes patients to infection with more virulent pathogens. Antineoplastic drugs (e.g., cyclophosphamide, doxorubicin, and fluorouracil) and acid-suppressive therapy (e.g., H2-receptor antagonists, proton-pump inhibitors, and antacids) also may result in changes in GI flora and possibly predispose patients to infection.1,3,13
Numerous factors, such as underlying disease, immunosuppressive drug therapy, and antimicrobial administration, determine the immunocompromised host’s risk of developing infection. Several risk factors are present concomitantly in many patients (see Table 100-1).
ETIOLOGY OF INFECTIONS IN NEUTROPENIC CANCER PATIENTS
![]() Infection remains a significant cause of morbidity and mortality in neutropenic cancer patients. More than 50% of febrile neutropenic patients have an established or occult infection.1,5 Patients with profound neutropenia are at greatest risk for systemic infection, with at least 20% of these individuals developing bacteremia.1,5 Areas of impaired or damaged host defenses, such as the oropharynx, lungs, skin, sinuses, and GI tract, are common sites of infection. These local infections may progress to cause systemic infection and bacteremia.5 Febrile episodes in neutropenic cancer patients can be attributed to microbiologically documented infection in approximately 30% to 40% of cases, about half of which are due to bacteremia. Further, infections can be documented clinically (but not microbiologically) in another 30% to 40% of patients, with the remaining 20% to 40% of patients manifesting infection only by fever.3,4,8,12
Infection remains a significant cause of morbidity and mortality in neutropenic cancer patients. More than 50% of febrile neutropenic patients have an established or occult infection.1,5 Patients with profound neutropenia are at greatest risk for systemic infection, with at least 20% of these individuals developing bacteremia.1,5 Areas of impaired or damaged host defenses, such as the oropharynx, lungs, skin, sinuses, and GI tract, are common sites of infection. These local infections may progress to cause systemic infection and bacteremia.5 Febrile episodes in neutropenic cancer patients can be attributed to microbiologically documented infection in approximately 30% to 40% of cases, about half of which are due to bacteremia. Further, infections can be documented clinically (but not microbiologically) in another 30% to 40% of patients, with the remaining 20% to 40% of patients manifesting infection only by fever.3,4,8,12
Table 100-1 lists organisms commonly infecting immunocompromised patients. Approximately 45% to 70% of bacteremic episodes in cancer patients are the result of gram-positive organisms compared with less than 30% of episodes documented during the 1970s and 1980s.1,4,7,12,14,15 This shift is attributed to the frequent use of indwelling central and peripheral IV catheters, frequent use of broad-spectrum antibiotics with excellent gram-negative activity but relatively poor gram-positive coverage, higher rates of mucositis caused by aggressive cancer treatments, and prophylaxis with trimethoprim–sulfamethoxazole or quinolones.1,4,7,12 Staphylococci (especially S. epidermidis) account for most infections, but Bacillus spp. and Corynebacterium jeikeium are also important pathogens.1,5,12,15 Rates of infection due to methicillin-resistant Staphylococcus aureus (MRSA) have increased in the hospital and community setting.16,17 Viridans streptococci, which may be resistant to β-lactams, also have emerged as important pathogens, particularly in patients with chemotherapy-induced mucositis of the oropharynx.4,12,18,19 Enterococci, including vancomycin-resistant strains, also may be problematic in many institutions.2,19 Bacteremia caused by vancomycin-resistant enterococci (VRE) in neutropenic patients is associated with a mortality rate exceeding 70%.4,20
Gram-positive infections do not always cause immediately life-threatening infections and are associated with somewhat lower mortality rates (approximately 5% to 10%) compared with gram-negative infections.1,12,15 However, increasing rates of antibiotic resistance have made treatment of gram-positive infections in immunocompromised patients more challenging.7,12 MRSA infections are associated with increased morbidity, mortality, and hospital costs compared with susceptible organisms.21 Methicillin resistance among coagulase-negative staphylococci, which may cause 40% to 80% of infections in certain populations, is common (70% to 90% of isolates).6,7,16 Organisms that are resistant to vancomycin are increasing in importance.1,5,7,19 Thus, prevention and timely diagnosis and treatment of gram-positive infections are clearly of great importance in the management of neutropenic cancer patients.
Gram-negative infections remain important causes of morbidity and mortality (approximately 20%) in immunocompromised cancer patients.12,15 However, the relative frequency of infection owing to specific pathogens has been shifting among gram-negative infections. E. coli and Klebsiella remain the most common isolates at many centers.15 Strains of Klebsiella producing plasmid-mediated extended-spectrum β-lactamases that hydrolyze extended-spectrum cephalosporins have emerged and are cause for concern.1,5,7,19 The frequency of infections resulting from other gram-negative organisms, such as Enterobacter, Serratia, and Citrobacter, has been increasing.1,5 Infections with these particular organisms may be difficult to treat because of the ease of β-lactamase induction and the more frequent development of resistance to multiple antibiotics.1,3,5,12,19
P. aeruginosa has long been an important pathogen in cancer patients. P. aeruginosa infection rates are decreasing in patients with solid tumors but not in patients with hematologic malignancies.4,7 Infections caused by P. aeruginosa are associated with significant morbidity and mortality in neutropenic patients, with mortality rates of 31% to 75% reported.12,15 The frequency of infection caused by difficult-to-treat organisms such as Stenotrophomonas maltophilia and Burkholderia cepacia appears to be increasing at many centers, probably because of selective pressures of broad-spectrum antimicrobial use.4,12 As with gram-positive organisms, antibiotic resistance among gram-negative organisms has continued to increase at alarming rates and has made appropriate antibiotic selection for treatment of febrile neutropenia more difficult.1,16 Although the GI tract is a common site of bacterial infection, severe infections caused by anaerobic organisms are relatively infrequent. Anaerobes are found most frequently in mixed infections, such as perirectal cellulitis and mucositis-associated oropharyngeal infections.12
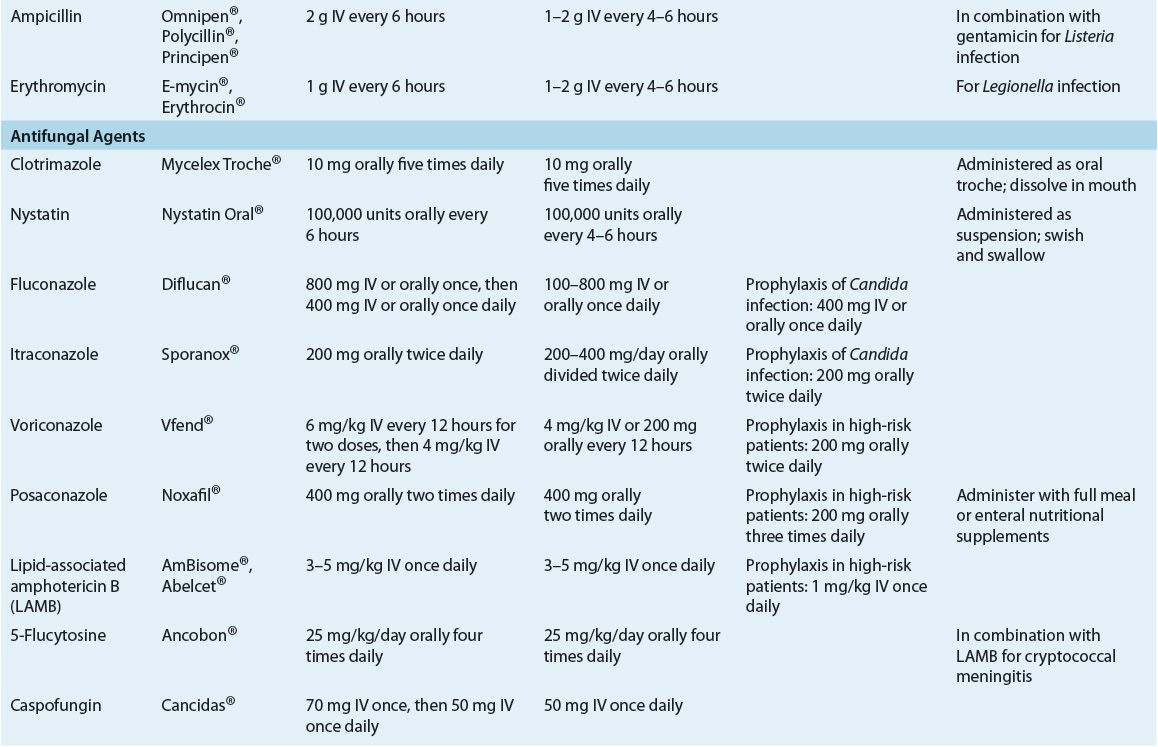
In addition to bacterial infections, neutropenic cancer patients are at risk for invasive fungal infections. Patients with extended periods of profound neutropenia who have been receiving broad-spectrum antibiotics, corticosteroids, or both are at the highest risk for invasive fungal infection. Up to one third of febrile neutropenic patients who do not respond to 1 week of broad-spectrum antibiotic therapy will have a systemic fungal infection.1,5,12 Large autopsy studies have documented that up to 40% of patients with hematologic malignancies had deep fungal infections, fully 75% of which were undiagnosed prior to death. Causative pathogens were usually either Candida spp. (35%) or Aspergillus spp. (55%).22
Candida albicans is the most common fungal pathogen in neutropenic cancer patients.4,12,23 However, non-albicans species of Candida including C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei are being isolated with increasing frequency and are more common than C. albicans infections in some studies.11,23 Increased infections caused by pathogens such as Trichosporon spp., Fusarium spp., and Curvularia have also been reported.23–25 The shift toward more frequent infection with non-albicans Candida is important because of significantly decreased rates of susceptibility among many of these strains.26 Because Candida spp. are normal flora, alteration of body host defenses is an important risk factor for the development of these infections. Oral thrush is the most common clinical manifestation of fungal infection. Mucous membranes damaged from chemotherapy and radiation serve as areas of Candida surface colonization and subsequent entry into the bloodstream; disease then may disseminate throughout the body. Organs such as the liver, spleen, kidney, and lungs are commonly involved in disseminated disease.22,24 Hepatosplenic candidiasis is a particularly important infection in patients with hematologic malignancies.3,22,24 Diagnosis of Candida infections is difficult and often requires invasive tissue sampling.6 In patients with invasive candidiasis, overall attributable mortality is as high as 35% to 50%.4,11,23
Invasive infections caused by Aspergillus are a serious complication of neutropenia, with mortality approaching 80% in patients with prolonged neutropenia and/or patients undergoing allogeneic HSCT.4,12 These infections are particularly prevalent in patients with hematologic malignancies and in patients undergoing HSCT.4,24,25,27 Infections resulting from Aspergillus species (including A. fumigatus, A. terreus, A. flavus, and A. niger) usually are acquired via inhalation of airborne spores. After colonizing the lungs, Aspergillus invades the lung parenchyma and pulmonary vessels, resulting in hemorrhage, pulmonary infarcts, and a high mortality rate. Invasive pulmonary disease is the dominant manifestation of infection in patients with neutropenia. However, Aspergillus also may cause other infections, including sinusitis, cutaneous infection, and disseminated disease involving multiple organs, including the CNS.27 Prolonged neutropenia is the primary risk factor for invasive pulmonary aspergillosis in patients with acute leukemia; use of corticosteroids also may predispose patients to disease.27 Invasive aspergillosis should be suspected in neutropenic cancer patients colonized with Aspergillus (in sputum and/or nasal cultures) who remain persistently febrile despite at least 1 week of broad-spectrum antibiotic therapy.1,5,27
Chemotherapy-induced mucous membrane damage may predispose neutropenic cancer patients to reactivation of HSV, manifesting as gingivostomatitis or recurrent genital infections. Untreated oropharyngeal HSV infections may spread to involve the esophagus and often coexist with Candida infections. Clinical disease resulting from HSV occurs most often in patients with serologic evidence (e.g., serum antibodies to HSV) of prior infection. Both HSV-seropositive HSCT patients and HSV-seropositive leukemics receiving intensive chemotherapy are at high risk for recurrent HSV disease during periods of immunosuppression.3,4,5,6,12
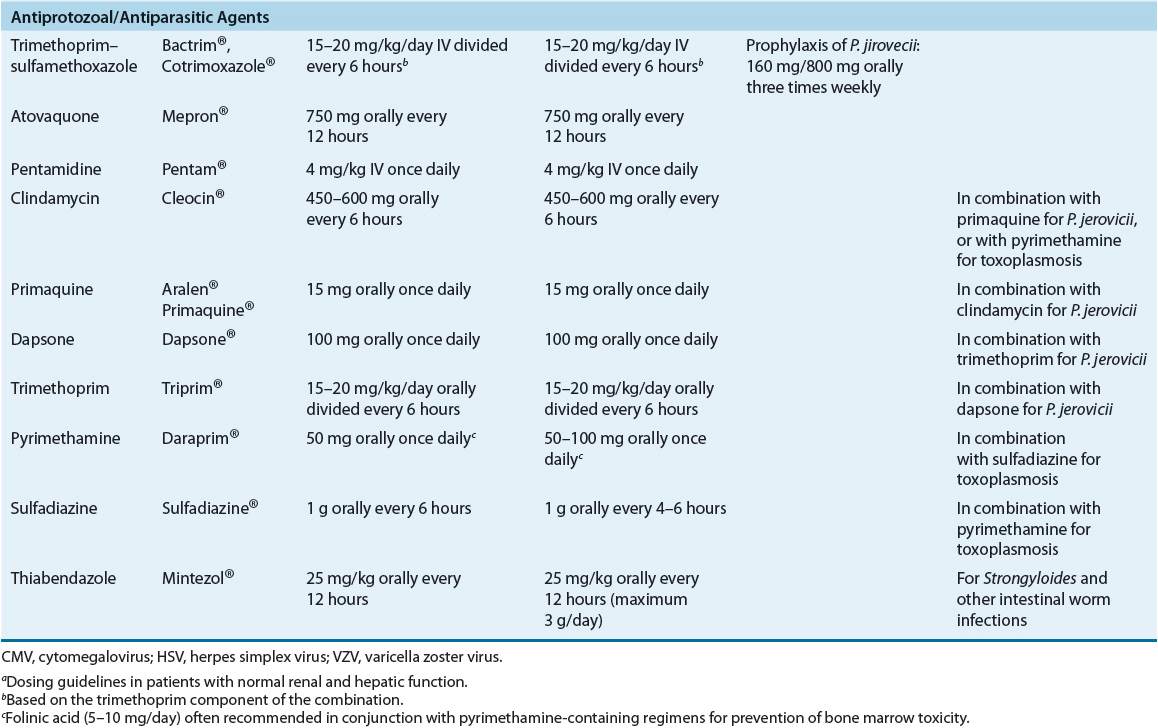
Pneumocystis jiroveci and Toxoplasma gondii are the most common parasitic pathogens found in immunocompromised cancer patients. Patients with hematologic malignancies (i.e., acute lymphocytic leukemia, lymphoma, and Hodgkin’s disease) and those receiving high-dose corticosteroids as part of chemotherapy regimens are at the greatest risk of infection.3,4,6,12 Routine use of trimethoprim–sulfamethoxazole prophylaxis has reduced substantially the incidence of these infections.1,5,6
Because the majority of infecting organisms in cancer patients are from the host’s own flora, some centers have used routine surveillance cultures in an attempt to prospectively identify causes of fever and suspected infection. In a typical surveillance culture program, cultures of the nose, mouth, axillae, and perirectal area are performed twice weekly, and culture results are correlated with the clinical status of the patient. Because these cultures are costly and have low diagnostic yield, the utility of surveillance culture programs is believed to be limited.1,5 However, surveillance cultures are useful as research tools and in patients with prolonged profound neutropenia and in institutions that have high rates of antimicrobial resistance or have problems with virulent pathogens such as P. aeruginosa or Aspergillus spp. Surveillance cultures should be limited to the anterior nares for detecting colonization with MRSA, Aspergillus, and penicillin-resistant pneumococci and to the rectum for detecting P. aeruginosa, multiple-antibiotic-resistant gram-negative rods, and VRE.1,12
Knowledge of infection rates and local susceptibility patterns is essential for guiding optimal management of febrile neutropenia. These parameters must be monitored closely because the spectrum of infectious complications is related to multiple factors, including cancer chemotherapy regimens and antimicrobial therapy used for treatment and prophylaxis.
CLINICAL PRESENTATION
![]() The most important clinical finding in the neutropenic cancer patient is fever. Because of the potential for significant morbidity and mortality associated with infection in these patients, fever should be considered to be the result of infection until proved otherwise.1–3,8,12 At the appearance of fever, the patient should be evaluated carefully for other signs and symptoms of infection.
The most important clinical finding in the neutropenic cancer patient is fever. Because of the potential for significant morbidity and mortality associated with infection in these patients, fever should be considered to be the result of infection until proved otherwise.1–3,8,12 At the appearance of fever, the patient should be evaluated carefully for other signs and symptoms of infection.
TREATMENT
Management of patients with febrile neutropenia, including both treatment and prophylaxis of infectious complications, can be extremely challenging. Although published guidelines are available, the most optimal clinical management of these patients remains unclear in many aspects.
Febrile Episodes in Neutropenic Cancer Patients
Desired outcomes
![]()
![]() The goals of therapy in neutropenic cancer patients with fever are the following: (a) protect the neutropenic patient from early death caused by undiagnosed infection; (b) prevent breakthrough bacterial, fungal, viral, and protozoal infections during periods of neutropenia; (c) effectively treat established infections; (d) reduce morbidity and allow for administration of optimal antineoplastic therapy; (e) avoid unnecessary use of antimicrobials that contribute to increased resistance; and (f) minimize toxicities and cost of antimicrobial therapy while increasing patient quality of life. Empirical broad-spectrum antibiotic therapy is effective at reducing early mortality.13
The goals of therapy in neutropenic cancer patients with fever are the following: (a) protect the neutropenic patient from early death caused by undiagnosed infection; (b) prevent breakthrough bacterial, fungal, viral, and protozoal infections during periods of neutropenia; (c) effectively treat established infections; (d) reduce morbidity and allow for administration of optimal antineoplastic therapy; (e) avoid unnecessary use of antimicrobials that contribute to increased resistance; and (f) minimize toxicities and cost of antimicrobial therapy while increasing patient quality of life. Empirical broad-spectrum antibiotic therapy is effective at reducing early mortality.13
CLINICAL PRESENTATION Febrile Neutropenia1,3,4,6
Approach to Treatment
General guidelines for management of febrile episodes and documented infections in neutropenic patients are shown in Figures 100-1 and 100-2.1 Although many controversies remain regarding optimal management of these patients, updated evidence-based guidelines from the Infectious Diseases Society of America (IDSA) for the management of febrile neutropenia were published in 2010.1 Similarly, the National Comprehensive Cancer Network (NCCN) published updated clinical practice guidelines for the prevention and treatment of cancer-related infections in 2012.5 Selected specific recommendations are discussed in the following sections of this chapter, and their associated evidence-based rankings are summarized in Table 100-2.
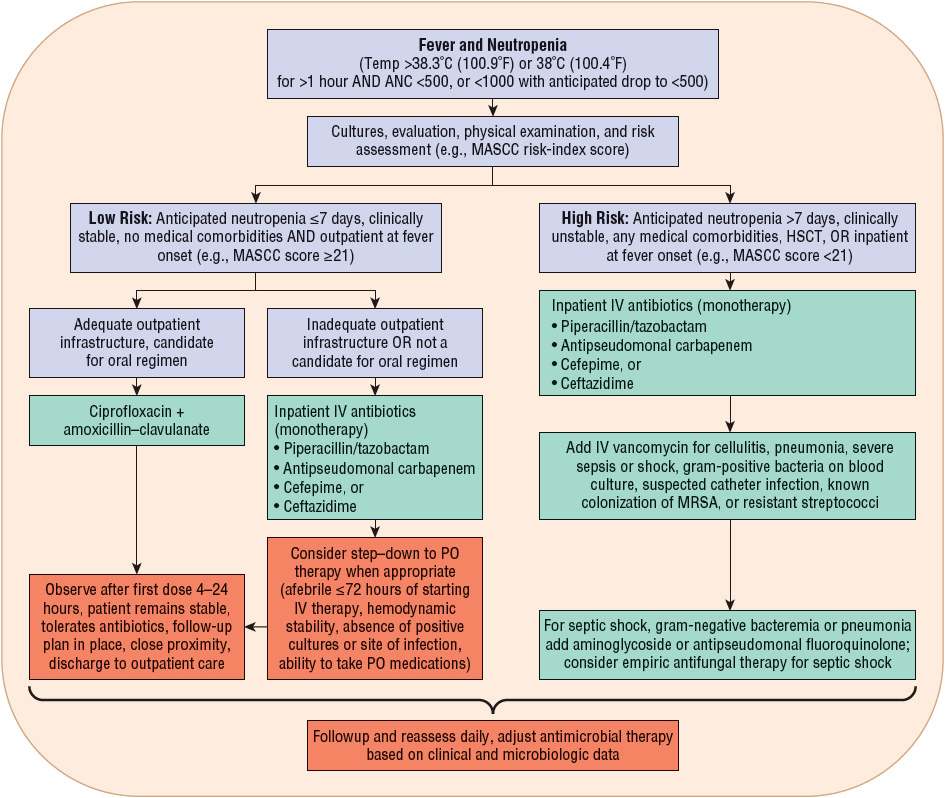
FIGURE 100-1 Initial management of febrile episodes in neutropenic patients. (ANC, absolute neutrophil count; HSCT, hematopoietic stem cell transplantation; MASCC, Multinational Association for Supportive Care in Cancer; PO, oral.)
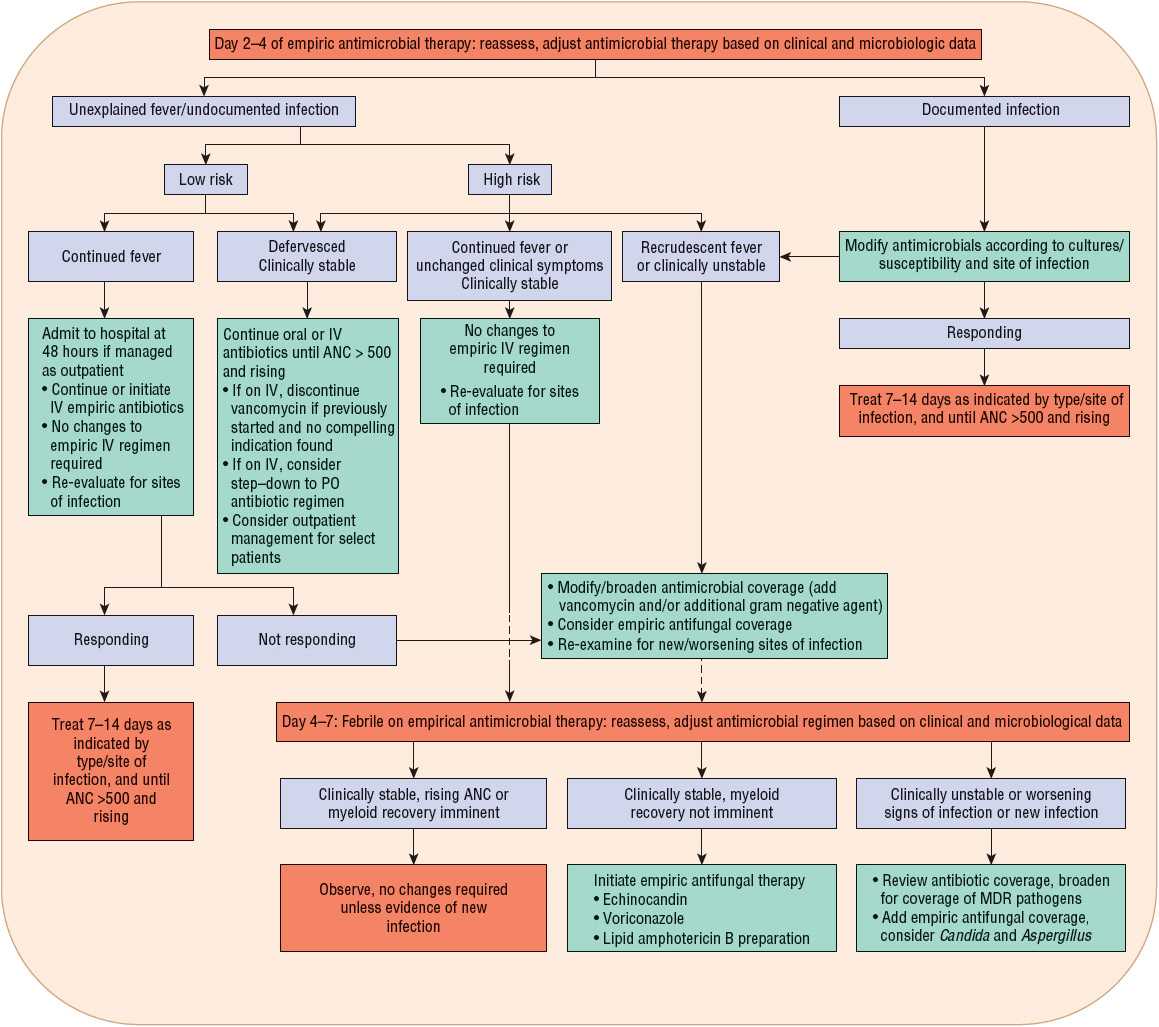
FIGURE 100-2 Subsequent management of febrile episodes in neutropenic patients who have already received empirical antimicrobial therapy for 2–4 days. (ANC, absolute neutrophil count; MDR, multidrug-resistant; PO, oral.)
TABLE 100-2 Summary of Evidence-Based Recommendations for Management of Febrile Episodes in Neutropenic Patients
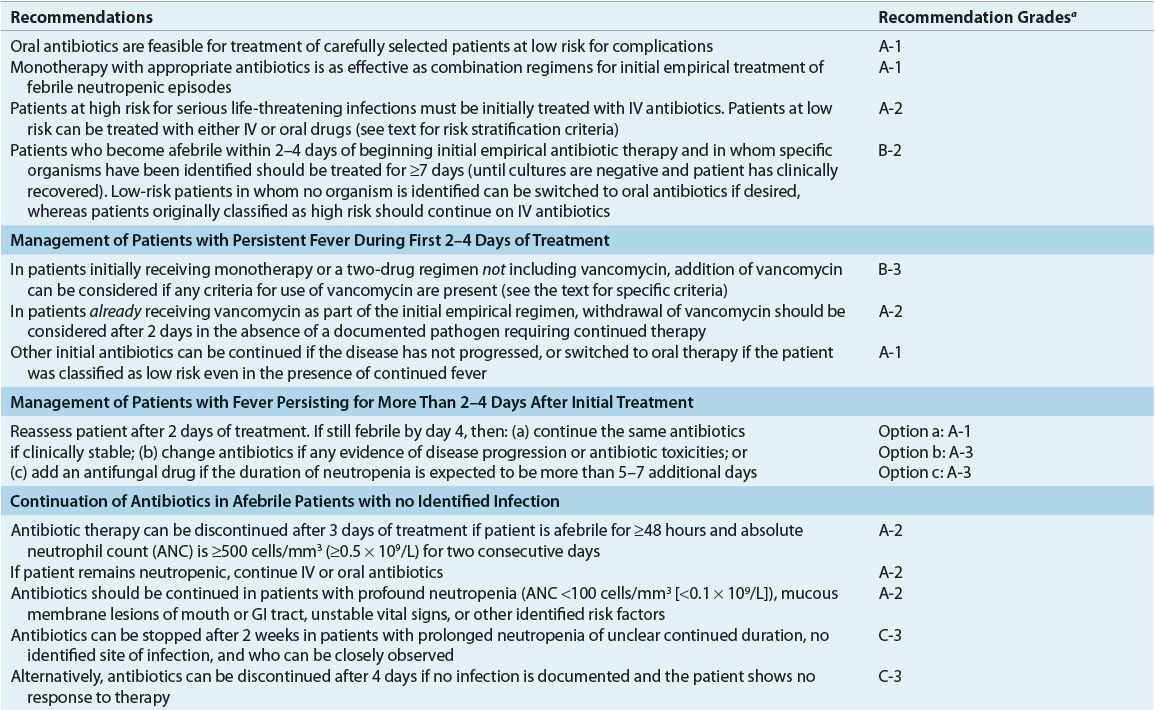
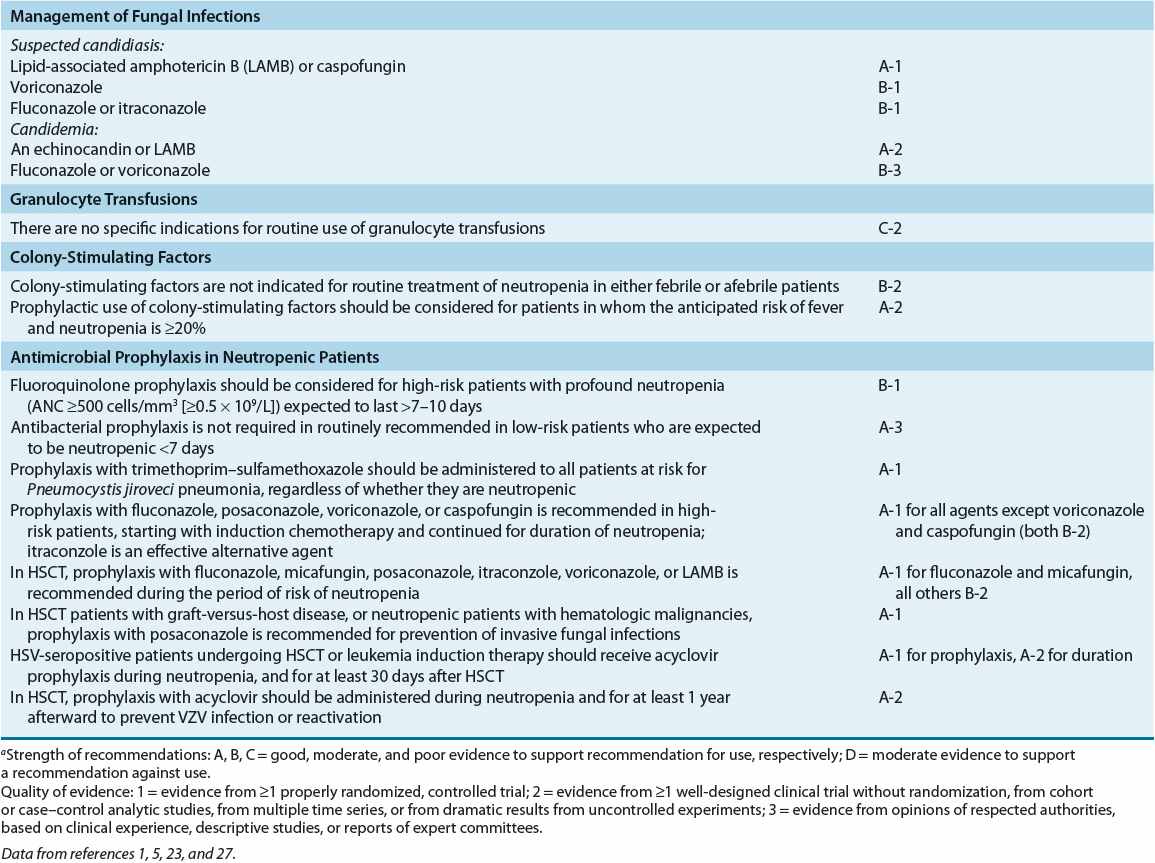
Fever in the neutropenic cancer patient is considered to be caused by infection until proved otherwise. High-dose broad-spectrum bactericidal, usually parenteral, empirical antibiotic therapy should be initiated at the onset of fever or at the first signs or symptoms of infection. Withholding antibiotic therapy until an organism is isolated results in unacceptably high mortality rates. Undiagnosed infection in immuno-compromised patients can rapidly disseminate and result in death if left untreated or if treated improperly. Failure to initiate appropriate antibiotic therapy for P. aeruginosa bacteremia at the onset of fever in neutropenic cancer patients resulted in mortality rates of 15% and 70% within 12 and 48 hours, respectively.1,5 Empirical antibiotic therapy is 70% to 90% effective at reducing early morbidity and mortality.1,5,7,12 Therapy must be appropriate and initiated promptly. Antimicrobial therapy must also be initiated promptly in afebrile cancer patients with clinical signs and symptoms of infection.
When designing optimal empirical antibiotic regimens, clinicians must consider infection patterns and antimicrobial susceptibility trends in their respective institutions. Patient factors such as risk for infection, drug allergies, concomitant nephrotoxins, and previous antimicrobial exposure (including prophylaxis) must be considered.1,4,5 Assessment of the patient’s risk of infection will help determine the appropriate route and setting for antibiotic administration (Fig. 100-1). Neutropenic patients with fever can be divided into low- and high-risk groups for complications of severe infection. Risk stratification drives both type and setting of antimicrobial therapy. The Multinational Association for Supportive Care in Cancer (MASCC) risk-index score is recommended by many clinical guidelines to assess a patient’s risk of complications.1,5 Most experts agree that, in general, low-risk patients have an anticipated duration of neutropenia ≤7 days, are clinically stable, and have no or few comorbidities and no bacterial focus or systemic signs of infection other than fever. In contrast, high-risk patients are those with an anticipated duration of neutropenia >7 days or profound neutropenia, are clinically unstable or have comorbid medical problems (e.g., focal or systemic signs of infection, GI symptoms, nausea, vomiting, diarrhea, hypoxemia, and chronic lung disease), or have a high-risk cancer (e.g., acute leukemia) and/or have undergone high intensity chemotherapy. High-risk patients (MASCC <21) should be hospitalized for parenteral antibiotics whereas low-risk patients may be candidates for oral or outpatient antibiotics. Even with such classifications, careful selection of low-risk patients for oral outpatient management is important (discussed in “Oral Antibiotic Therapy for Management of Febrile Neutropenia” section below).1,5,28–30
The optimal antibiotic regimen for empirical therapy in febrile neutropenic cancer patients remains controversial, but it is clear that no single regimen can be recommended for all patients. Because of their frequency and relative pathogenicity, P. aeruginosa and other gram-negative bacilli and staphylococci remain the primary targets of empirical antimicrobial therapy.1,12 Although P. aeruginosa is documented in fewer than 5% of bloodstream infections in the population of hospitalized patients, adequate antipseudomonal antibiotic coverage still must be included in empirical regimens because of the significant morbidity and mortality associated with this pathogen.1,4,12,16 All empirical regimens must be carefully monitored and appropriately revised on the basis of documented infections, susceptibilities of bacterial isolates, development of more defined clinical signs and symptoms of infection, or a combination of these factors.
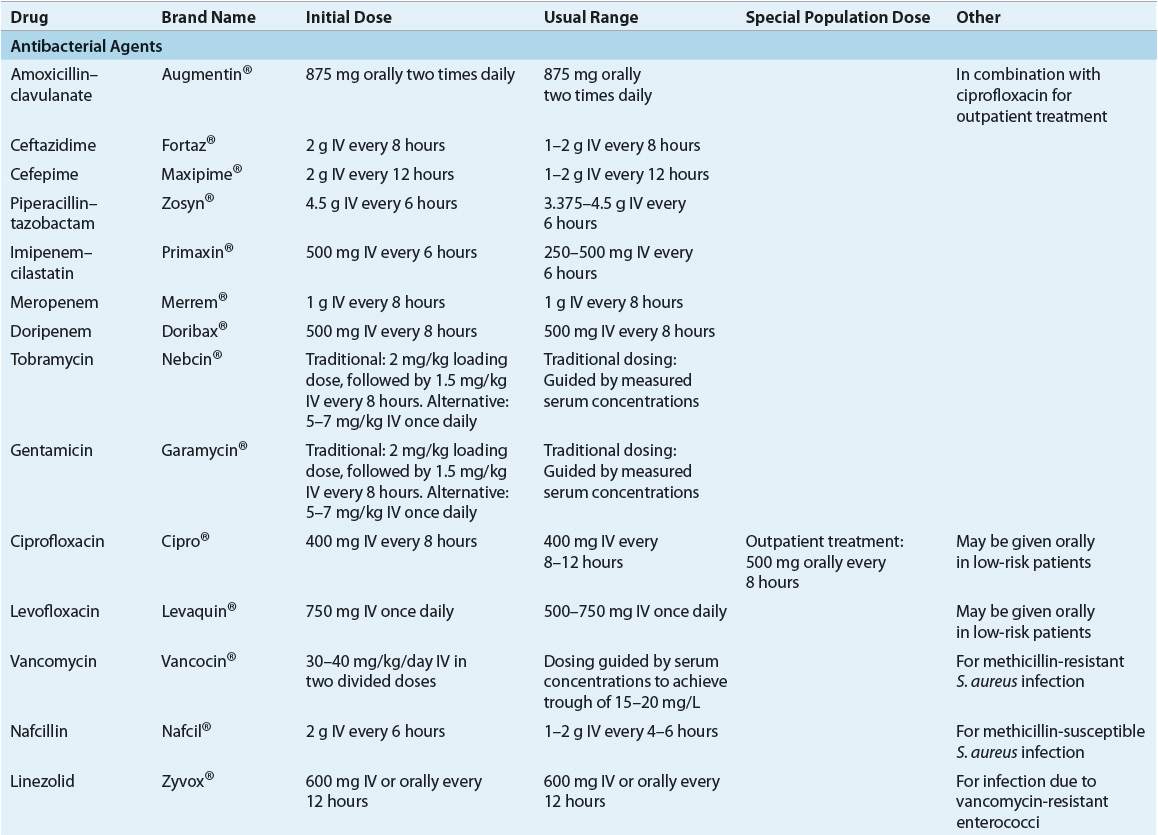
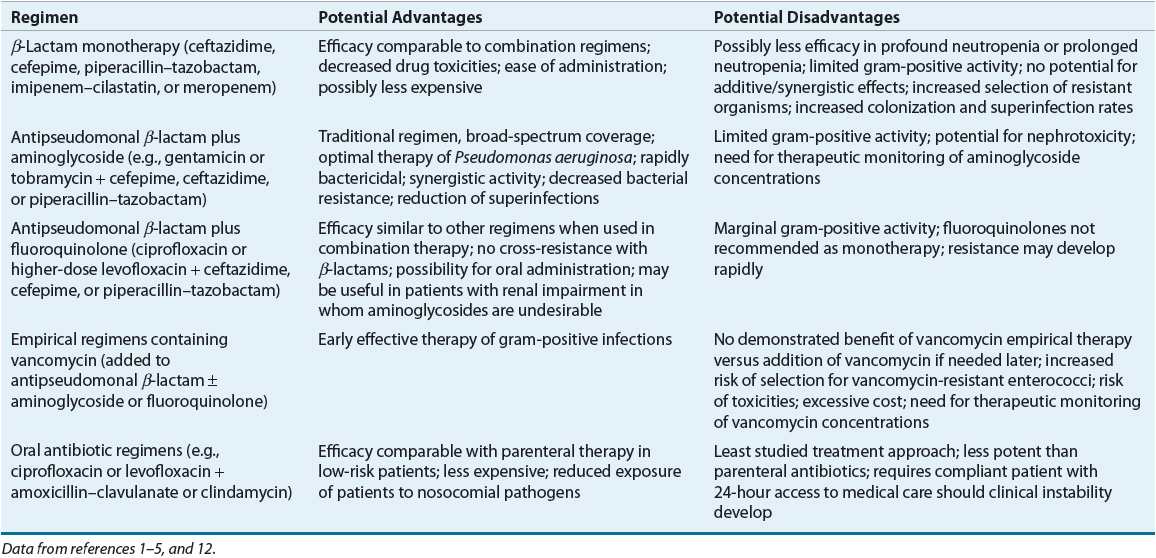
Although there are some differences among them, consensus guidelines generally recognize three different types of empirical parenteral antibiotic regimens: (a) monotherapy with an antipseudomonal β-lactam such as a cephalosporin (cefepime or ceftazidime), a carbapenem (imipenem–cilastatin, meropenem, or doripenem), or piperacillin–tazobactam; (b) two-drug combination therapy with an antipseudomonal β-lactam plus either an aminoglycoside or an antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin); and (c) monotherapy or two-drug combination therapy as above, plus the addition of vancomycin (Fig. 100-1).1,5 Each of these regimens has advantages and disadvantages, which are summarized in Table 100-3. There is no overwhelming evidence that any one of these regimens is superior to the others. The overall response to empirical antibiotic regimens in febrile neutropenic cancer patients is approximately 70% to 90% regardless of whether a pathogen is isolated or which antimicrobial regimen is used.1,4,5,7,12 Additionally, other alternative regimens may also appropriate based on specific patient characteristics or susceptibilities of suspected pathogens.
TABLE 100-3 Comparative Advantages and Disadvantages of Various Antibiotic Regimens for Empiric Therapy of Febrile Neutropenic Cancer Patients
β-Lactam Monotherapy
Monotherapy with an antipseudomonal β-lactam is recommended by IDSA 2010 and NCCN 2012 guidelines as initial parenteral therapy for management of febrile neutropenia without suspected or proven resistant organisms or complications (e.g., pneumonia, hypotension, vascular access infection, etc.)1,5 Several β-lactam antibiotics in current use have been evaluated as monotherapy for management of febrile episodes in neutropenic cancer patients, including antipseudomonal cephalosporins (ceftazidime and cefepime), piperacillin–tazobactam, and antipseudomonal carbapenems (imipenem–cilastatin and meropenem).1,3,5,12,14 Three different meta-analyses assessing as many as 46 clinical trials involving more than 7,600 patients found no significant differences overall between monotherapy and combination therapy (β-lactam/aminoglycoside) in rates of survival, treatment response, and bacterial/fungal superinfections.31–33 One study also found a higher rate of adverse effects in aminoglycoside-containing combination regimens.32 In addition, one analysis found that cefepime monotherapy was associated with a significantly higher risk of mortality compared with the other β-lactams evaluated.1,5,33 A follow-up analysis conducted by the FDA using additional studies and patient-level data failed to confirm an increased risk of mortality with cefepime, concluding that it is as efficacious as other β-lactams.1,5,34 Significantly lower response rates for ceftazidime (but not cefepime) monotherapy have been reported in another review of the clinical literature.1,5 However, until the results of these studies can be validated, ceftazidime is still are among the monotherapy regimens routinely recommended as appropriate initial therapy of febrile neutropenic patients, although with a lower strength of evidence in 2012 NCCN guidelines.1,5,12,33,34 Institutional susceptibility patterns and patient characteristics should drive drug selection.
Doripenem has an appropriate overall spectrum of antibacterial activity with good activity against P. aeruginosa and other gram-negative organisms as well as many gram-positive pathogens. Neither the 2012 NCCN nor the 2010 IDSA consensus guidelines specifically recommend doripenem as appropriate for monotherapy due to a lack of supportive clinical evidence at the time the guidelines were written.1,5 Doripenem is, however, considered by many clinicians to be appropriate for this use.
Use of monotherapy has several potential advantages and disadvantages (see Table 100-3). Perhaps the most common concerns are those regarding the selection of resistant strains of organisms, such as P. aeruginosa, Enterobacter spp., and Serratia spp., through extended-spectrum β-lactamases and type 1 β-lactamases, especially with ceftazidime.1,5,7,12,19 Activity against gram-positive organisms such as coagulase-negative staphylococci, MRSA, enterococci (including VRE), penicillin-resistant S. pneumoniae, and some strains of viridans streptococci is poor with some single β-lactams, but cefepime and antipseudomonal carbapenems have good activity against viridans streptococci and pneumococci.1,5 Although ceftazidime has been studied widely and used for treatment of febrile neutropenia, newer agents may be more effective owing to ceftazidime’s susceptibility to β-lactamase induction and lower activity against gram-positive organisms.1,7,19,33 Ertapenem, a carbapenem, and tigecycline, a glycylcycline antibiotic, have excellent activity against many gram-negative organisms but should not be used in the empirical treatment of febrile neutropenia due to their weaker activity against P. aeruginosa.
As with all empirical antibiotic regimens, patients receiving monotherapy should be monitored closely for treatment failure, secondary infections, and development of resistance. Use of mono-therapy may not be appropriate in institutions with high rates of gram-positive infections or infections caused by relatively resistant gram-negative pathogens such as P. aeruginosa and Enterobacter. The carbapenems are less susceptible to inducible β-lactamases and often may be used effectively in these institutions. Overall, similar efficacy has been observed with monotherapy with antipseudomonal β-lactams compared to aminoglycoside combination therapy for treatment of P. aeruginosa infections.1,5,12,32,33
Aminoglycoside Plus Antipseudomonal β-Lactam
Regimens consisting of an aminoglycoside plus an antipseudomonal β-lactam traditionally have been the most commonly used for empirical treatment of febrile neutropenia, although many such regimens may lack adequate gram-positive activity (see Table 100-3).1,5 This relative lack of activity remains a concern because of the increasing frequency of gram-positive infections. The choice of aminogly-coside and β-lactam for inclusion in empirical regimens should be based on institutional epidemiology and antimicrobial susceptibility patterns. Similar efficacy is observed with an antipseudomonal β-lactam in combination with an aminoglycoside.1,5
Combinations of broad-spectrum β-lactams and aminoglycosides often provide synergistic activity against bacteria commonly infecting neutropenic patients. The exact role of synergy in the outcome of febrile neutropenic patients treated with empirical antibiotic therapy is somewhat controversial, particularly in light of the efficacy of single-drug regimens. Nevertheless, synergistic combinations of antibiotics appear to be beneficial in patients with persistent profound neutropenia. Moreover, administration of antipseudomonal β-lactams in combination with an aminoglycoside may result in a lower rate of drug resistance.4
Aminoglycoside toxicity may be a concern in patients receiving these regimens who are already receiving other nephrotoxic drugs, such as cisplatin and cyclosporine. Administration of aminoglycosides in large single daily doses (once-daily dosing) may be as effective, less costly, and no more toxic than conventional dosing methods.41 Although once-daily aminoglycoside dosing regimens appear to be safe and effective in these patients, standard dosing regimens are recommended for infections where data are not sufficient to recommend once-daily dosing (e.g., endocarditis).1,5,12
Fluoroquinolones as a Component of Empirical Regimens
Because the fluoroquinolone antibiotics have broad-spectrum activity (particularly against gram-negative pathogens), rapid bactericidal activity, and favorable pharmacokinetic and toxicity profiles, these agents have been investigated as empirical therapy for febrile neutropenic patients. Ciprofloxacin is the preferred agent for use in this clinical setting because of its relatively better activity against P. aeruginosa and more extensive evidence-based support for its use.1,12 Response rates to quinolone-containing combination regimens are comparable to those obtained with the other regimens described previously.1,4,5 Ciprofloxacin is not recommended for monotherapy, however, because of its relatively poor activity against gram-positive pathogens, particularly streptococci, and variable response rates in clinical studies.1,5 Fluoroquinolones should also not be used as empirical therapy in patients who have received quinolones as infection prophylaxis because of the risk of drug resistance.1,5,12 Rates of fluoroquinolone resistance are increasing, and streptococcal treatment failures are a concern.16,19 Although fluoroquinolones are not generally considered first-line empirical therapy, they may be useful as one component of combination regimens in patients with allergies or other contraindications to first-line agents.1,5
Empirical Regimens Containing Vancomycin
The inclusion of vancomycin in initial empirical therapy of febrile neutropenic cancer patients is not currently recommended by IDSA 2010 or NCCN 2012 guidelines unless the patient has specific risk factors; however, this remains an ongoing debate. This controversy continues because of the increasing incidence of gram-positive infections in this population, particularly MRSA. One approach is to include vancomycin in the initial empirical antibiotic regimen, thereby providing early effective treatment of possible gram-positive infections. Inclusion of vancomycin in initial empirical regimens may be more appropriate today because of higher rates of MRSA infections as well as aggressive chemotherapy regimens causing significant mucosal damage that increases the risk for streptococcal infections. Decreased mortality from penicillin-resistant viridans streptococcal infections has been observed when vancomycin was included in initial therapy.1,5,12 A second approach is to withhold vancomycin from initial empirical regimens, later adding the drug if gram-positive organisms are isolated from cultures or if there is no response to initial therapy. Support for both these approaches can be found in the medical literature.1,5,12,35,36 Prospective studies and at least two meta-analyses have failed to document increased response rates or decreased mortality with the routine addition of vancomycin to initial empirical regimens, provided that vancomycin can be added later as needed.1,5,12,35,36 In addition to increased costs of therapy, vancomycin was also associated with increased adverse effects, including nephrotoxicity.35,36 Finally, concerns remain regarding selection of resistant gram-positive bacteria such as VRE with excessive vancomycin use.1,5,12
Vancomycin is currently recommended for inclusion in initial empirical regimens only in patients at high risk for gram-positive infection, particularly due to MRSA and coagulase-negative staphylococci (including patients with evidence of infection of central venous catheters and other indwelling lines), high risk for viridans streptococcal infection due to severe mucositis, or pneumonitis or soft tissue infection in hospitals with high rates of MRSA infections.1,3,5,7,12,35,36 Rates of β-lactam resistance among viridans streptococci range from 18% to 29%.5 Empirical vancomycin use may be justified in institutions using empirical or prophylactic antibiotic regimens without good activity against streptococci (e.g., ciprofloxacin) and in patients known to be colonized with MRSA or β-lactam–resistant pneumococci. In patients with preliminary culture results indicating gram-positive infection, empirical vancomycin is appropriate while the susceptibility results are pending. Lastly, empirical use of vancomycin may be recommended in patients with hypotension or other evidence of cardiovascular impairment or sepsis without an identified pathogen.1,5,35,36 If empirical vancomycin therapy is initiated and no evidence of gram-positive infection is found after 48 to 72 hours, the drug should be discontinued.1,4,5 Continuing vancomycin when not warranted results in higher costs, more toxicities, and greater risk of development of VRE.1,5
Other antimicrobial agents, such as quinupristin–dalfopristin, linezolid, daptomycin, telavancin, and ceftaroline, should be reserved for documented infections caused by multiresistant gram-positive pathogens that are not susceptible to, or are unresponsive to, vancomycin. The role of these drugs in the routine treatment of fever in neutropenic patients is undetermined, and linezolid is associated with myelosuppression.1,5
Oral Antibiotic Therapy for Management of Febrile Neutropenia
An individual patient’s risk for complications of severe infection determines appropriate antibiotic therapy and the proper setting for administration (see Table 100-3).1,4,5 Risk stratification is based on several parameters (e.g., MASCC score as mentioned above) as well as response to empirical antimicrobial therapy if IV therapy is initially given.1 Because of the excellent spectrum of activity and favorable pharmacokinetics of currently available oral antibiotics, particularly the fluoroquinolones, oral antibiotics have an important role in the management of selected patients. In patients at low risk for severe or complicated bacterial infection, empirical therapy with broad-spectrum oral antibiotic agents achieves similar patient outcomes as parenteral antibiotics, with response rates of 77% to 95%.1,4,12,28–30 This has made possible the treatment of febrile neutropenia in low-risk patients in the outpatient setting. Patients judged to be low risk with reliable follow-up may be appropriate candidates for oral antibiotic therapy administered on an outpatient basis.1,4,12,28–30 Ciprofloxacin in combination with amoxicillin–clavulanate (or clindamycin for penicillin-allergic patients) for enhanced gram-positive coverage has been most commonly studied for outpatient therapy in low-risk patients and is recommended by IDSA and NCCN guidelines.1,5 In general, monotherapy with ciprofloxacin should be avoided due to relatively poor gram-positive activity. Levofloxacin has been used as monotherapy for outpatient treatment of low-risk patients, due to enhanced gram-positive activity; however, this regimen has not been well studied and is not formally recommended by IDSA or NCCN guidelines. If used, only the higher-dose levofloxacin 750 mg regimen should be administered in order to provide adequate activity against organisms such as P. aeruginosa.1,5 Careful patient selection obviously is required for such management strategies. Important criteria include patient and provider comfort, a history of medication compliance, good caregiver support, a follow-up plan, and close proximity, prompt access and transportation to appropriate medical care around the clock in the event of failure to respond to outpatient antibiotic therapy. If a patient qualifies for oral therapy based on social and clinical status, the first dose of oral regimen should be given and the patient observed for 4 to 24 hours to ensure tolerance and the patient remains clinically stable. Benefits of oral therapy on an outpatient basis include increased convenience and quality of life for patients and caregivers and reduced exposure to multidrug-resistant institutional pathogens.1,5 Outpatient therapy of low-risk patients now is common practice in most institutions.
In patients at low risk for severe bacterial infection who were initiated on IV antibiotics, oral antibiotics may play a role in step-down therapy. Carefully selected neutropenic patients may be safely switched from broad-spectrum parenteral therapy to oral antibiotic regimens (e.g., ciprofloxacin plus amoxicillin–clavulanate) with response rates comparable to patients remaining on IV therapy.12,28–30 Patient selection criteria generally include defervescence within 72 hours of initiation of parenteral therapy, hemodynamic stability, absence of positive cultures or a discernible site of infection, and ability to take oral medications. Many of these patients are able to complete their course of therapy at home.1,5,12,28–30 Changing parenteral antimicrobials to oral regimens in carefully selected patients is now relatively common practice and allows for less expensive hospitalizations and earlier patient discharges.
Antimicrobial Therapy After Initiation of Empirical Therapy
![]() After initiation of empirical antimicrobial therapy (Table 100-4), judicious assessment of febrile neutropenic cancer patients is mandatory to evaluate response, clinical status, laboratory data, and potential need for therapy adjustments. After 2 to 4 days of empirical antimicrobial therapy, the clinical status and culture results of febrile neutropenic patients should be reevaluated to determine whether therapeutic modifications are necessary (Fig. 100-2). Modifications of antimicrobial therapy should be based on clinical and laboratory data; antibiotic therapy should be optimized based on culture results. However, during periods of neutropenia, patients generally should continue to receive broad-spectrum therapy because of risk of secondary infections or breakthrough bacteremias when antimicrobial coverage is too narrow.1,5,12 The treatment duration for a documented infection should be appropriate for the particular organism and site, and should continue for at least the duration of neutropenia (until ANC ≥500 cells/mm3 [≥0.5 × 109/L]) or longer if clinically necessary.
After initiation of empirical antimicrobial therapy (Table 100-4), judicious assessment of febrile neutropenic cancer patients is mandatory to evaluate response, clinical status, laboratory data, and potential need for therapy adjustments. After 2 to 4 days of empirical antimicrobial therapy, the clinical status and culture results of febrile neutropenic patients should be reevaluated to determine whether therapeutic modifications are necessary (Fig. 100-2). Modifications of antimicrobial therapy should be based on clinical and laboratory data; antibiotic therapy should be optimized based on culture results. However, during periods of neutropenia, patients generally should continue to receive broad-spectrum therapy because of risk of secondary infections or breakthrough bacteremias when antimicrobial coverage is too narrow.1,5,12 The treatment duration for a documented infection should be appropriate for the particular organism and site, and should continue for at least the duration of neutropenia (until ANC ≥500 cells/mm3 [≥0.5 × 109/L]) or longer if clinically necessary.
< div class='tao-gold-member'>