Implant Pathology
Key Facts
Terminology
Breasts may be augmented or replaced by foreign material for cosmetic reasons or for reconstruction after surgery
Foreign material (such as silicone, paraffin, or organic oils) may be directly injected into breast tissue
This procedure is associated with high rate of complications
Majority of implants consist of thin silicone shell filled with saline or silicone gel
Tissue expanders are saline implants that are placed temporarily before tissue reconstruction
Implants may be placed within the breast anterior to pectoralis muscle or below the muscle
Clinical Issues
Implants are associated with a variety of complications
Capsular contracture is most common complication and may require surgery to correct
Silicone shell thins and may rupture; silicone can migrate to lymph nodes and distant sites
Infection may occur, usually during perioperative period
Capsule may calcify; this, and a thin delicate shell, can make mammographic screening more difficult
Rare cases of fibromatosis have been associated with implants
Very rare cases of T-cell lymphomas are reported in association with implants
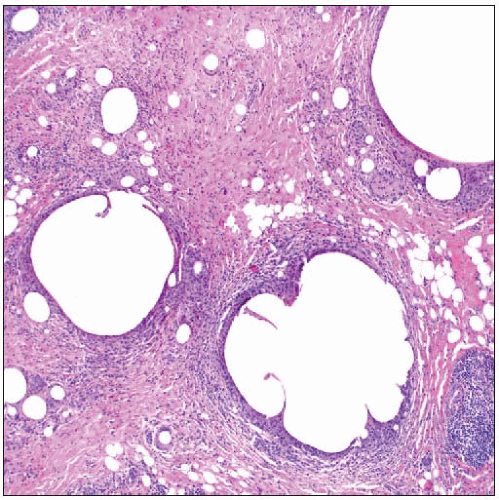
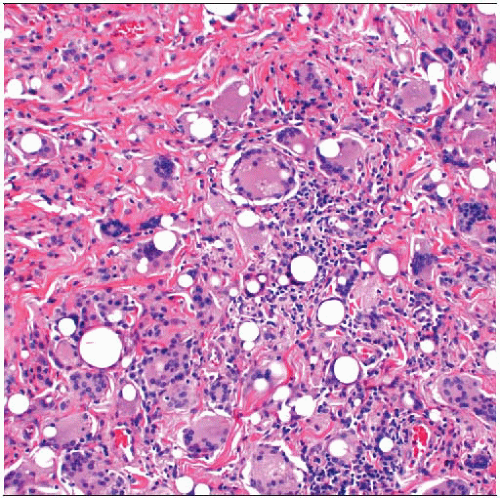 Thick fibrotic capsules form around breast implants and are associated with various types of foreign material. It may not be possible to determine the composition of many implants. |
TERMINOLOGY
Synonyms
Silicone implant
Saline implant
Tissue expanders
Definitions
Foreign material placed within chest wall to replace or enhance breast tissue
Some implants are placed between breast tissue and pectoral muscle
Subpectoral implants are placed between pectoral muscle and chest wall
ETIOLOGY/PATHOGENESIS
Types of Implants
Direct injection of substances has been used to augment breast size
Substances used have included organic oils, silicone, paraffin, and others
These substances usually migrate over time and result in a poor cosmetic appearance
This is not an accepted medical procedure
Foreign material can closely mimic a malignancy on breast imaging
Material may cause skin or nipple retraction and migrate to lymph nodes causing lymphadenopathy
Saline implants
These implants have thin silicone outer shell that is filled with saline
Some are intended for permanent use
Tissue expanders are temporary saline implants used prior to definitive breast reconstruction
Usually have a port that can be used to inject more saline
Implant is eventually replaced by permanent implant or by tissue reconstruction
If saline implant ruptures, there is immediate deflation and saline is quickly resorbed by surrounding tissue
Silicone implants
Silicone implants have thin silicone outer shell and are filled with silicone gel
These implants are intended for permanent use
If silicone implant ruptures, silicone is usually confined within surrounding fibrotic capsule
Imaging studies may be necessary to detect ruptured implant
Implants with polyurethane patches
Rough surfaced patches were used on some older implants to reduce effects of fibrotic response
Polyurethane has specific histologic appearance in capsular tissue
History of Breast Implants
1st silicone shell implant was used in 1961
From 1992-2006, use of silicone implants was restricted to FDA-approved research projects due to safety concerns
Many well-publicized lawsuits were based on claims that implants were associated with autoimmune-type diseases
In 1999, National Institute of Medicine released a report stating that connective tissue disease was not more common in women with implants
In 2006, FDA lifted the moratorium on implants but did require follow-up studies of patients
In 1992, 32,000 women underwent augmentation procedures
In 2007, 347,000 underwent augmentation procedures
There are likely 2,000,000-5,000,000 women in USA currently with breast implants
CLINICAL ISSUES
Presentation
Infections associated with implants are rare
Most cases occur in perioperative period and are due to skin bacteria
In very rare cases, unusual organisms have been reported (fungi or mycobacteria)
Treatment
If contracture is pronounced, implant may need to be replaced to achieve acceptable cosmetic result
In the past, contractures were treated with pressure to “break” the capsule
This technique is no longer used as it may result in leakage of silicone into adjacent breast tissue
If infection occurs, implant must be removed
Complications and Treatment
Silicone implant rupture and migration of silicone
Silicone can “bleed” through intact implant shell and may be present in surrounding capsule
Silicone is usually restricted to capsule when implant is intact
If both implant and capsule are ruptured, silicone can migrate to distant sites
Most common site is regional lymph nodes
Silicone has also been reported to migrate to distant subcutaneous tissue and lung
Implant-associated lymphoma
May occur with both saline and silicone implants
Usually presents as implant complication, such as seroma or presumed infection
Presents 1-23 years after surgery
About 1/2 of patients have had cosmetic surgery and 1/2 breast reconstruction after breast cancer
Majority of implant-associated lymphomas arise from T cells
Only 10% of all primary breast lymphomas are T-cell lymphomas
22 of 30 cases of primary breast anaplastic large cell lymphomas have been associated with implants
Implant-associated mesenchymal tumors
Most common type of soft tissue tumor occurring in breasts with implants (either silicone or saline filled) is fibromatosis
Tumor is generally detected with 2-3 years of surgery
These women are not known to have mutations in adenomatosis polyposis coli gene or to have Gardner syndrome
Sarcomas occurring in women with implants are extremely rare and are of diverse types
Specific etiologic relationship between tumor formation and implants has not been established
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree