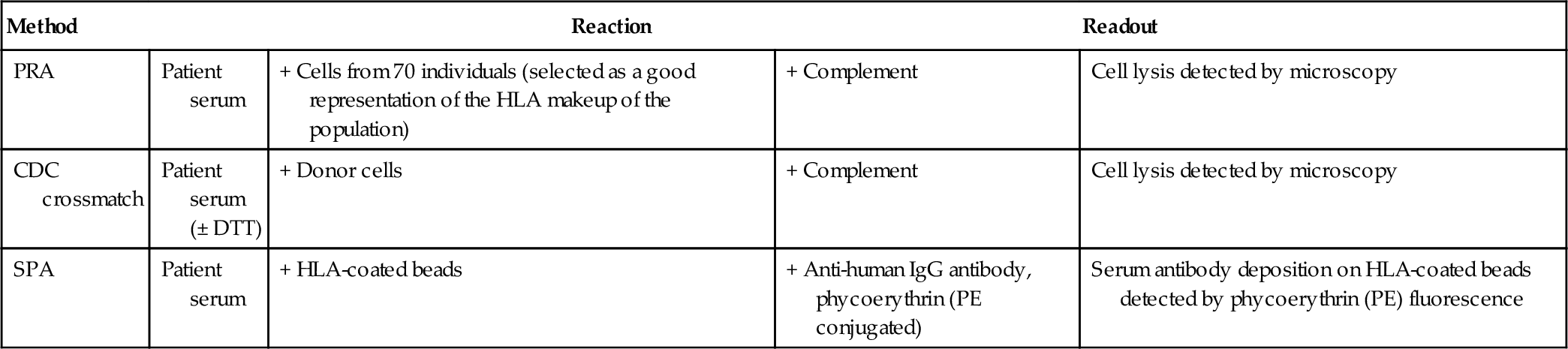
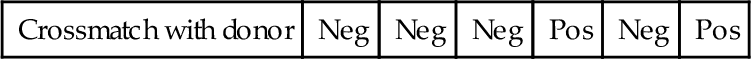Chapter 15 George Vlad; Raphael Clynes; Adriana I. Colovai; Elena-Rodica Vasilescu A. Complement-dependent cytotoxicity (CDC) against a reference panel of T and B cells. B. Flow cytometry (FC) crossmatch with donor peripheral blood mononuclear cells and panel reactive antibody (PRA) testing. C. Solid-phase assays (SPAs). D. CDC against a reference panel of T cells and B cells and SPAs. 2a. Consider the following CDC and FC crossmatch results shown in Table 15-1. Which crossmatch result presents the highest risk of rejection? B. Scenario B. C. Scenario C. D. Scenario D. 2b. Referring to the same alloantibody testing methods and results described above, which set of crossmatch results requires additional testing? B. Scenario B. C. Scenario C. D. Scenario D. 2c. Referring to the same alloantibody testing methods and results described above, which positive crossmatch is due to an IgM antibody? B. Scenario B. C. Scenario C. D. Scenario D. 3. A kidney transplant patient is treated with pulse corticosteroids and rituximab after acute mediated rejection is detected as outlined by the Banff criteria (graft dysfunction, C4d deposition detected in the biopsy, and circulating alloantibodies). The patient eventually undergoes nephrectomy. Although the level of circulating anti–human leukocyte antigen (HLA) antibodies was low during the rejection episode (mean fluorescence intensity [MFI] ~ 2000, by SPAs), the level continues to rise steadily. After nephrectomy, the MFI values reach 10,000 at day 7 and 12,000 at day 21. Which one of the following accounts for this phenomenon? B. Anti-donor HLA antibodies continue to be produced after nephrectomy; however, the graft no longer adsorbs them. C. The readings are an artifact of the rituximab treatment, which can persist in circulation many months after the treatment. D. The histocompatibility lab switched reagent lots, and the new reagents are much better than the old ones at detecting the antibodies against the specific allele of the donor in this case. 4. A family typing at the HLA-A/-B/-DR loci is shown in Table 15-2. Which one of the following children has HLA gene recombination? B. Child 2. C. Child 3. D. Child 4. 5. A patient who underwent successful kidney transplantation 20 years ago loses his allograft to chronic rejection. He is listed for retransplantation. His HLA typing performed at a different institution shows the following results: A. Splits of HLAs, indistinguishable by old serologic typing methods. B. Egregious errors of the previous histocompatibility lab. C. Egregious errors of the current histocompatibility lab. D. The donor’s HLAs are detected in the patient’s blood. 6. A patient with end-stage renal disease who is highly sensitized is considered for an antibody reduction protocol with plasmapheresis and intravenous immunoglobulin (IVIg). Which one of the following parameters should determine the number of plasmapheresis courses to be administered? A. Number of mismatched antigens. B. Number of previous transplants. C. The PRA percentage. D. Titer of donor-specific antibodies. 7. A transplantation patient who is not presensitized to any HLAs receives a 5/6 matched allograft. The typing results are as follows: B. The patient produced multiple anti-HLA antibodies against mismatched, nontyped loci (HLA-C, -DQ, -DP), which cross-react with multiple HLA-A and -B alleles. C. The patient produced antibodies against the β-2 microglobulin chain of HLA class I expressed by half of the cell panel and used by all the HLA alleles identified by SPAs. D. The patient produced a single antibody against the epitope Bw4 present in HLA-B27 and many HLA-A and -B alleles. 8. In kidney transplantation, living related donors represent an important source of donated organs and tend to have significantly better outcomes. A distinct advantage of donation from a relative is the possibility of good donor-recipient antigen matching; first-degree related donors (i.e., parents, children) might share one haplotype with the recipient. However, not all donor-recipient pairings are created equal. Which one of the following is the worst pairing among first-degree relatives? B. Father donates to child. C. Child donates to mother. D. Child donates to father. 9. Which one of the following is a prediction of compatibility based on the patient’s alloantibody status compared with specific donors’ antigens? B. Direct crossmatch. C. Virtual crossmatch. D. Flow crossmatch. 10. A heart transplant candidate is broadly presensitized, displaying antibodies against more than 80% of the cell panel (PRA > 80%). The antibody specificities established by SPAs include the common HLA-A2, present with a frequency of more than 40% in many human populations; however, this is not listed as an unacceptable antigen due to the apparently low amounts of this antibody (MFI = 3800). He is desensitized using plasmapheresis and IGIg and receives an HLA-A2 + heart on the basis of a negative crossmatch. A few months after transplantation, he experiences a severe infection with Streptococcus pneumoniae. Immunosuppression is lowered and the infection clears with antibiotics. However, after this episode the patient describes heart failure symptoms and the ejection fraction seems to have significantly decreased compared with the preinfection measurements. Antibody monitoring shows that multiple anti-HLA non–donor-specific antibodies are sharply upregulated, although the only donor-specific antibody (anti–HLA-A2) is disappearing (MFI = 1100) (Figure 15-1). Which one of the following is the best explanation for these observations? A. Graft dysfunction is caused by a thrombus. B. Graft dysfunction caused by a humoral response against the pathogen, which cross-reacts with non-HLA heart antigens. C. Antibody-mediated rejection caused by complement-fixing non–donor-specific antibodies that cross-react with donor HLA alleles. D. Antibody-mediated rejection caused by the anti–HLA-A2 antibody, which increased along with other anti-HLA antibodies as a consequence of the infection. 11. In which one of the following situations would HLA allele-level typing be most useful for the identification of an HLA-identical sibling? A. Mother: DR1 DR15; father: DR3, DR7. B. Mother: DR1 DR3; father: DR4, DR7. C. Mother: DR1 DRX; father: DR1, DR4. D. Mother: DR11 DR4; father: DR3, DR7. 12. The results of a CDC crossmatch are indicated in Table 15-3. Which one of the following conclusions can be drawn? B. The serum contains a donor-specific IgG antibody and a blocking IgM antibody. C. The serum contains a donor-specific IgM antibody and a blocking IgG antibody. D. The serum contains a donor-specific IgA antibody. Table 15-3 13. Which one of the following features is shared by HLA class I and II molecules? A. Types of T cells they stimulate. B. Types of cells that express them. C. The chromosome on which they are located. D. Types of antigens they present. E. Types of cells that contain a β-2 microglobulin subunit. 14. Which one of the following describes the reaction of an antibody with antigens that are similar, but not identical, to the antigen that stimulated antibody production? B. Cross-reactivity. C. Memory response. D. Mixed lymphocyte reaction. E. PRA. 15. Which one of the following types of serum antibodies is detected by standard FC crossmatch performed with donor lymphocytes? A. Anti-donor HLA class I IgG. B. Anti-donor HLA class I and class II IgG. C. Anti-donor non-HLA IgG. D. Anti-donor lymphocyte IgG. E. Cytotoxic anti-donor HLA IgG and IgM. 16. In which one of the following situations is high-resolution HLA class I and class II typing most important? A. Living related kidney transplantation in patients with high PRA. B. Liver transplantation. C. Stem cell transplantation: unrelated donor. D. Stem cell transplantation: two haplotype–matched related donor. E. ABO-incompatible renal transplantation. 17. Which one of the following laboratory tests involves direct recognition of alloantigens by T lymphocytes? B. CDC crossmatch. C. HLA typing using sequence-specific oligonucleotides (SSOs). D. Mixed lymphocyte culture. E. Mitogen T-cell stimulation. 18. As part of the immunologic evaluation of a renal transplant candidate, anti-HLA antibody screening was performed by CDC and SPA. Using an HLA-typed cell panel, CDC screening indicated that no PRAs (PRA = 0%) were present in the patient’s serum. However, SPA using single HLA-coated beads revealed the presence of serum IgG antibodies reactive to HLA-A2, -A28, -A9, and –B17. These results indicate which one of the following? A. There is a high probability that the CDC crossmatch with an HLA-A2 donor will be positive. B. There is an increased risk of acute antibody-mediated rejection if the patient received an HLA-A2 transplant. C. There is a high risk of hyperacute rejection if the patient received an HLA-A2 transplant. D. Transplantation with an HLA-A2 graft is contraindicated even if the CDC crossmatch is negative. E. The results of the CDC and solid-phase immunoassays are discordant and, therefore, they are invalid. 19. Which one of the following statements about lymph nodes is correct? A. Dendritic cells enter the germinal centers through high endothelial venules. B. Long-lived plasma cells reside primarily in the germinal center and paracortical areas. C. Antigen-independent proliferation of B cells occurs in lymph nodes. D. Activated T cells are absent from germinal centers. E. Affinity maturation of B cells occurs in the germinal center. 20. A kidney transplant patient receiving chronic immunosuppressive medication presents with lymphadenopathy. He was Epstein-Barr virus (EBV) negative before transplantation and received an allograft from an EBV-positive donor. FC of a biopsy shows both Ig κ and λ staining of the B-cell gate. Which one of the following is true? A. The patient is not at risk for EBV lymphoma. B. EBV reactivation in immunocompromised patients is due to humoral insufficiency and can be overcome with IVIg treatment. C. B-cell proliferation in EBV lymphoproliferative disorders is due to B-cell receptor–mediated B-cell recognition of EBV antigens. D. The receptor for EBV viral entry on B cell is the complement receptor CD21. E. EBV lymphoma is less commonly seen in pediatric transplants since posttransplant lymphoproliferative disorder is due to EBV reactivation from prior infection. 21. A patient with systemic lupus erythematosus (SLE) is awaiting a kidney transplant. The FC crossmatch with a potential donor is positive both on T cells and B cells, but so is the auto-crossmatch. Which one of the following is the best course of action in this case? B. Rule out this donor because the crossmatch is positive regardless of the auto-crossmatch results. C. Do not transplant. These are autoantibodies (auto-crossmatch–positive) that recognize the same antigen on donor cells and, therefore, will destroy the transplanted kidney as well. D. Perform SPA testing to determine whether the antibodies are anti-HLA antibodies, and if yes, whether they are donor specific. 22a. The HLA-A locus typing of a patient who is a bone marrow transplant candidate and two potential donors are shown in Table 15-4. Which donor is “the best” match? A. Donor 1 due to the allele mismatch with the patient, A*03:01 vs. A*03:04. B. Donor 2 due to the antigen mismatch with the patient, A*03:01 vs. A*01:01. C. No difference between donors 1 and 2 regarding the impact of mismatch on graft survival. D. Depends on where the mismatched amino acid(s) lie in the HLA structure. Table 15-4 22b. Would a third donor (donor 3) typing as A*02:01, A*02:305 N be a better choice than donors 1 and 2? A. Yes. Donor 3 is better than donor 2, but equivalent to donor 1. B. No. Donor 3 has an exotic allele, which is highly likely to elicit allorecognition and trigger graft-versus-host disease (GVHD). C. Yes. Donor 3 has a perfectly matched allele A*02:01 and a null allele A*02:305 N, which does not contribute to the allorecognition. D. No. Homozygosity or single-allele expression at one locus in the donor may trigger GVHD. 23. The likelihood of a mother and her daughter being HLA identical (assuming no consanguinity in the family) is closest to which one of the following percentages? B. 50%. C. 25%. D. 16.7%. E. 0%. 24. Which one of the following is true about antigen presentation by HLA molecules? A. There are an unlimited amount of peptides that can be presented from a single protein. B. HLA haplotypes can contribute to immune responsiveness to a specific virus or vaccine. C. Only extracellular antigens can be presented on HLA class II molecules. D. Only intracellular antigens can be presented on HLA class I molecules. E. Only the amino acid sequence determines the immunogenicity of peptides loaded onto HLA molecules. 25. A transplant recipient with swollen glands, high fever, and fatigue presents to the emergency department. A peripheral smear of the blood shows high numbers of “atypical” lymphocytes and a second sample is sent for FC. Which one of the following statements is true? B. In infectious mononucleosis, the atypical lymphocytes are likely a large number of activated CD8+ T cells. C. B-cell leukemia can be excluded if the atypical cells are surface B-cell receptor negative. D. EBV-positive serology is seen in around 50% of adults. E. Stable integration of the EBV genome into chromosomal DNA of infected B cells is required for latency. 26. Which one of the following statements is true regarding the alloreactive cellular infiltrates? B. T-cell production of interferon (IFN)-γ, IL-12, and IL-17 augments the cellular immune response. C. Cyclosporine blocks T-cell receptor signaling via inhibition of the tyrosine kinase nuclear factor of activated T cells (NFAT). D. Toll-like receptors recognize only microbial constituents and activate innate immune cells through necrosis factor (NF)- κB. E. Regulatory T cells have the capacity to induce tolerance by killing self-reactive effector T cells. 27. Which one of the following explains why an individual does not normally make an immune response to a self-protein? A. Self-proteins cannot be processed into peptides. B. Peptides from self-proteins cannot bind to HLA class I molecules. C. Peptides from self-proteins cannot bind to HLA class II molecules. D. Lymphocytes reactive to self-proteins are inactivated by deletion, anergy, or receptor editing. E. Developing lymphocytes cannot rearrange V genes required to produce a receptor for self-proteins. 28. A patient with active rheumatoid arthritis feels ill with low-grade fever, malaise, morning stiffness, and fatigue. Which one of the following proteins or cytokines is most likely responsible for these symptoms? B. Rheumatoid factor. C. Tumor necrosis factor (TNF) and IL-1. D. IL-4 and IL-10. 29a. A patient with end-stage renal disease (ESRD) is evaluated for a living unrelated kidney transplant. The results of histocompatibility testing by SPAs (Luminex with mixed antigen beads) and CDC and FC crossmatches between patient sera and patient lymphocytes and between patient sera and donor lymphocytes are shown in Table 15-5. Which one of the following best explains these results? B. An HLA class I antibody. C. An HLA class II antibody. D. An autoantibody. E. Major histocompatibility complex (MHC) class I polypeptide-related sequence A (MICA) antigen antibody. 29b. The results of the pretransplant crossmatch are shown in Table 15-6. The transplant proceeds and the patient undergoes induction therapy with rituximab. If the testing were repeated 1 week after transplant, what would the results most likely show? 29c. Which one of the following tests offers additional information about the anti-HLA class I antibody present in this serum, complementing the testing results described in the previous question? B. Luminex/SPA with single antigen beads. C. T- and B-cell CDC crossmatch with anti-human globulin. D. High-resolution typing of the recipient and donor. 30. Which one of the following diseases is associated with HLA-DQB1*06:02? B. Celiac disease. C. Ankylosing spondylitis. D. Behçet’s disease. 31. In transplantation, the T-cell contribution to allograft rejection is caused primarily by direct recognition of the donor’s MHC molecules by clonal T-cell receptors (TCRs). This strong recognition is based on which one of the following? A. Recipient peptides presented on donor MHC class II molecules to recipient T cells. B. Complementarity-determining regions (CDRs) of recipient TCR stably binding the polymorphic domains of the donor MHC. C. Stable binding of CD4 co-receptor molecules to nonself MHC class II alleles. D. Stable binding of CD8 co-receptor molecules to nonself MHC class I alleles. E. Stable binding of both CD4 co-receptor molecules to nonself MHC class II alleles and stable binding of CD8 co-receptor molecules to nonself MHC class I alleles. 32. Which one of the following treatments used in transplantation can cause a false-positive FC crossmatch? B. Rituximab. C. Prednisone. D. Mycophenolate mofetil. 33. Assuming there are no recombination hot spots in the HLA chromosome region, which HLA gene pair displays the highest recombination frequency (Figure 15-2)? B. HLA-B and HLA-DR. C. HLA-DR and HLA-DQ. D. HLA-A and HLA-DR. 34. An allograft transplant recipient who received a kidney from an older HLA-identical brother (HLA-A/B/C/DR typed, 8/8 identity) is experiencing graft dysfunction 4 months after transplantation. A biopsy reveals cellular infiltrates with the majority being T cells. Which one of the following is the most likely basis of T-cell reactivity in this case? B. Crossover event at the HLA-DQ locus. C. Age of the donated organ. D. The direct recognition pathway. E. The indirect recognition pathway. 35. According to current (2011) national transplant statistics published by the United Network for Organ Sharing (UNOS), heart, kidney, and liver primary allograft survival rates are roughly 70% at 5 years. Which one of the following is true regarding lung allograft survival? A. It is much better than other solid organs because the lung is an immune-privileged site. B. It is much worse than other solid organs due to the inflammation elicited by airborne antigens. C. It is about in line with other solid organs because rejection is histocompatibility driven and other organ-specific factors play only minor roles. D. All lung allografts are lost within 5 years due to bronchiolitis obliterans syndrome (BOS). 36. Which one of the following molecular HLA designations shows the correct use of nomenclature to identify a particular allele? B. DRB1*030101. C. DRB1*03:01:01. D. DRB103:01:01. 37. Hyperacute rejection distinguishes itself from other types of acute and chronic rejection through the extreme alacrity of its onset. Immunologically, the speed of this reaction is explained by its reliance on which one of the following? A. Preformed donor-specific antibodies and complement deposition. B. Preformed donor-specific antibodies and alloreactive T cells. C. Preformed donor-specific antibodies, de novo antibody production against the graft, and complement deposition. D. De novo antibody production against the graft and complement deposition. 38a. SPAs are the newest methods used in histocompatibility testing for alloantibody detection. SPA tests for alloantibodies rely on which one of the following? A. Beads coated with antigen(s). B. Magnetic beads. C. FC. D. Anti-human globulin (AHG)–augmented CDC crossmatch. 38b. Which one of the following can cause interference in the identification of anti-HLA antibody specificity by SPA (Luminex)? A. Cells with poor viability binding antibodies nonspecifically. B. Absence of the target allele on testing beads. C. Instrument sensitivity. D. Assay interference by medications. 39. Which one of the following is an advantage of SPAs with single antigen beads in histocompatibility testing? A. Identification of complement fixing antibodies. B. Prediction of a negative effect on graft survival. C. Identification of HLA specificities of antibodies. D. Prediction of acute cellular rejection (ACR) risk. 40. A kidney transplant candidate underwent a thorough pretransplant antibody study. Serum testing on a panel of cells from 70 individuals (PRA) shows that her serum lyses five (7%) of the cells, all of which are HLA-B27+. SPAs performed on the same serum indicate that the patient has two antibodies against HLA-B27 and HLA-B35, and they both present roughly similar MFI values (6000-7000). A child of the patient (who is HLA-B27+) and a sibling (who is HLA-B35+) volunteer for donation. The CDC crossmatch reactions with the child’s cells (HLA-B27+) are positive in the presence or absence of dithiothreitol (DTT), but the CDC crossmatch with the HLA-B35+ sibling’s cells is negative. Which one of the following is the best explanation for this observation and the best recommendation for transplantation (Table 15-7)? B. The anti–HLA-B35 is an IgG1 or IgG3 antibody, and transplantation with the sibling’s kidney is not acceptable. C. The anti–HLA-B27 is an IgG antibody and transplantation with the child’s kidney is an acceptable option. D. The anti–HLA-B35 is an IgG2 or IgG4 antibody, and transplantation with the sibling’s kidney is an acceptable option. 41. A female leukemia patient received a bone marrow allograft. She is blood type O + and shows high titers of anti-A and anti-B antibodies. Her donor is a male of blood type AB +. At 3 months posttransplant, the patient has the following data entered into her medical record: 100% white blood cells (WBCs) of donor origin by karyotyping Y chromosome–carrying cells. The recipient ABO testing yields O + results. Which one of the following is the likely explanation for these results? A. The WBC karyotyping is wrong due to interference by anti-donor (ABO) antibodies. B. The ABO typing was performed on a different patient’s sample. C. The ABO results were obtained by back typing and front typing should be performed. D. Both results are correct; donor bone marrow produces WBCs while recipient bone marrow produces red blood cells. 42. Posttransplant de novo anti-HLA antibodies develop in an allograft recipient with no history of sensitization (pretransplant). Which one of the following can be caused by these de novo antibodies? B. Recurrence of the original disease. C. Hyperacute rejection episodes. D. Chronic rejection. 43. Which one of the following statements is true? B. Anti-CD25 monoclonal antibody therapy can reduce alloreactive B-cell responses. C. Administration of IVIg can increase the titer of alloreactive antibodies. D. Bortezomib is a proteosome inhibitor that induces apoptosis by accumulation of large amounts of protein. 44. Which one of the following includes all of the generally accepted signs of antibody-mediated rejection of a solid organ allograft? B. Circulating donor-specific antibodies, Cd4 deposition in the graft, and graft dysfunction. C. Donor-specific antibodies, C4d deposition in the graft, and ejection fraction less than 40%. D. Biopsy IgG immunofluorescence, C4b deposition in the graft, and graft dysfunction. Major points of discussion ■ PRA testing performed by CDC detects IgG and IgM complement fixing antibodies. The pattern of reaction against T cells and/or B cells can hint to the nature of the anti-HLA antibodies. T-cell–positive and B-cell–positive reactions signal the presence of anti-HLA class I antibodies, whereas T-cell–negative and B-cell–positive reactions reveal anti-HLA class II antibodies. PRAs can detect antibodies against other (non-HLA) antigens if they are expressed on the cells used for testing. ■ PRA cannot detect the presence of antibodies that do not fix complement. Although the HLA typing of the cells forming a panel is known, it is sometimes impossible to ascertain the specificities of the antibodies in the serum of a highly sensitized patient (i.e., PRA > 90%). ■ SPAs use collections of beads coated with mixtures or individual HLAs. This allows resolution of the antibodies’ HLA specificities. SPAs have a limit of detection that surpasses that of any other test currently used. SPAs also allow a relative quantification of the antibodies by the mean fluorescence intensity (MFI) readouts. ■ SPAs also have several limitations. Generally, only HLAs are coated onto SPA beads; therefore, non-HLA antibodies cannot be detected. SPAs are not designed to detect IgM antibodies. It cannot distinguish between complement fixing and nonfixing antibodies. ■ PRA and SPA technologies complement each other nicely, as each method provides information not available from the other.4,21,22 2a. A. Scenario A. 2b. A. Scenario A. 2c. A. Scenario A. Major points of discussion ■ CDC crossmatch can detect complement fixing IgG and IgM antibodies against donor cells. ■ FC crossmatch is designed to detect the binding of IgG to donor cells but not their complement fixing ability. ■ Anti-HLA class I antibodies in serum will give T-cell– and B-cell–positive crossmatch reactions due to the expression of HLA class I (i.e., HLA-A, -B, -C) on all cells. ■ Anti-HLA class II antibodies in serum will give only B-cell–positive crossmatch reactions since only antigen-presenting cells (APCs) such as B cells express HLA class II antigens (i.e., HLA-DR, -DQ, -DP). ■ No HLA distribution explains a T + B– crossmatch reaction. Certain anti–T-cell antibody treatments often used in transplantation (such as Orthoclone OKT3, ATG) may, however, result in a T + B– crossmatch. 3. A. Nephrectomy was incomplete; residual tissues left behind continue to be rejected and generate a humoral response. Major points of discussion ■ Rituximab can persist in the circulation of patients for months and can interfere with some antibody testing methodologies. As an anti–B-cell antibody, rituximab causes false-positive results in CDC assays using B cells. ■ SPAs, which use beads coated with specific antigens (usually HLAs), have several advantages, including the following: • Ability to resolve antibody specificity (if an anti-HLA antibody) • Immunity to interference from most monoclonal antibody therapies currently used since those target antigens are not coated onto the beads ■ SPAs also suffer from a few shortcomings: • Inability to distinguish harmful complement fixing antibodies from non–complement fixing ones, • Inability (using the standard SPA procedures) to identify antibody isotypes other than IgG • Inability to detect antibodies to non-HLAs1,19,22 4. A. Child 1. Major points of discussion ■ The frequency of crossover between two loci is directly proportional to the distance between the two genes on the chromosomes. ■ The genetic identity between a parent and a child is exactly 50%. ■ There is a 25% chance that siblings will be 100% HLA matched or 100% mismatched by inheriting the same or the other parental HLA haplotypes. There is a 50% chance that siblings will be 50% HLA matched. ■ Chromosomal crossover events can yield unexpected HLA matching between siblings, such as 8/10 HLA matching (when 0/10, 5/10, or 10/10 identity is expected). Allele-level matching is extremely important in bone marrow transplantation, and crossover events bear great importance in donor selection. ■ Family studies aim to establish the parental haplotypes by tracking which HLA alleles “travel” together. 5. A. Splits of HLAs, indistinguishable by old serologic typing methods. Major points of discussion ■ It was realized early on that some anti-HLA antibodies recognized multiple HLAs, hence the need to “split” what was formerly thought to be a single antigen into newly discovered subtypes, that is, B16 → B38, B39; B17 → B57, B58. ■ With the advent of genetics, HLA loci were discovered to be much more polymorphic than could ever be classified by serology. For example, at the genetic level, HLA-B57 appeared to be a collection of alleles B*57:01, B*57:02, etc. ■ In this nomenclature convention (i.e., B*57:01), the first two numbers represent the serologically defined antigen and the second two represent the allelic subtype. ■ Finally, in 2011 a new nomenclature was introduced to address the problem that arises at loci that have more than 99 genetically defined alleles—that is, HLA-B*5799 would be the last allele in which the genetic resolution can be described by two digits. The newest nomenclature looks like HLA-B*57:01:02, and the last two digits define alleles of B*57:01 with silent mutations. ■ Patients with long allograft survival times may have typing results in their charts and medical records conforming to outdated nomenclatures.9,20 6. A. Number of mismatched antigens. Major points of discussion ■ A cytotoxicity crossmatch is a useful tool in screening against such reactions. ■ However, there are such highly sensitized patients for whom a donor is nearly impossible to find; that is, the patient will have at least one antibody against any combination of HLAs. Without desensitization protocols, these patients’ access to lifesaving organ transplantation would be blocked or severely limited. ■ Desensitization protocols, usually relying on plasmapheresis and IVIg, can be used to reduce the titers of antibodies present in the patient’s circulation and inhibit the production of more antibodies from plasma cells. ■ If the titers of antibodies against a donor can be lowered sufficiently so as not to yield a positive CDC crossmatch, the patient may be safely transplanted. 7. A. The patient produced donor-specific antibodies to a likely mismatched allele of HLA-A2 (which is present in about 40% of the population) not represented on the SPA beads but cross-reacts with multiple HLA-A and -B alleles. Major points of discussion ■ The Bw4 antigen consists of four amino acids at positions 80-83 in HLA class I molecules. It is present on alleles HLA-B5 (51, 52, 5102, 5103), 0802, 0803, 13, 17 (57, 58), 1809, 27, 37, 38 (16), 44 (12), 47, 49 (21), 53, 5607, 59, 63, 67, 77, and HLA-A23, 24, 25, 32. ■ The Bw6 epitope consists of 4 amino acids: NLRG 80-83 at positions 80-83 in HLA class I molecules. It is present on alleles HLA-B7, 703, 8, 1309, 18, 2708, 2712, 2718, 35, 39 (16), 3901, 3902, 4005, 4406, 4409, 45 (12), 46*, 4702, 48, 50 (21), 54 (22), 55 (22), 56 (22), 60 (40), 61 (40), 62 (15), 64 (14), 65 (14), 67, 71 (70), 72 (70), 73, 75 (15), 76 (15), 78, 81. ■ Only a handful of HLA-B alleles have neither Bw4 nor Bw6 (HLA-B1806, -B4601, -B5503, and –B7301). ■ A transplant recipient who has only one Bw epitope can form antibodies against the other Bw epitope. ■ Bw4/6 antibodies can have a major impact on the donor pool available to the presensitized patient. 8. A. Mother donates to child. Major points of discussion ■ A main component of central tolerance is the negative selection of self-reactive T cells and B cells in the thymus and bone marrow. Positive selection of self-cognate T cells also occurs, ensuring that T and B cells possess functional T-cell receptors and B-cell receptors, respectively, and giving rise to T regulatory cells. ■ Maternal antigens crossing the placental barrier can participate in the central tolerance being established in the fetus, thus accounting for better mother-to-child allograft survival. ■ Fetal antigens crossing the placental barrier cannot participate in the central tolerance establishment in the mother, which by the time of the pregnancy is largely shut down. Thus, the mother’s adult immune system recognizes these antigens as exogenous and can create antibodies to them. ■ Pregnancy constitutes a well-documented allosensitizing event. 9. A. Auto-crossmatch. Major points of discussion ■ The technology consists of an ELISA reaction where the antigen(s) are fixed on a solid phase (microbeads). A laser-based detection system allows quantification of the amount of antibody detected based on MFI readings. ■ An important caveat of the technology is that, although it provides accurate information on the antibodies’ specificities and quantity, it does not provide any qualitative information. Most importantly, it does not detect the complement-fixing ability of the antibodies detected. ■ A virtual crossmatch relies on SPA measurements of the circulating antibodies in a transplant patient, then refusing donor offers from individuals who express HLAs to which antibodies were detected. ■ This method carries the risk of denying access to transplantation of terminally ill patients who, although having high titers of alloantibodies in circulation, do not have complement-fixing antibodies and, therefore, may fare well with a negative CDC crossmatch transplant. 10. A. Graft dysfunction is caused by a thrombus. Major points of discussion ■ Antibody testing methods test the levels/titers of circulating antibodies. ■ Organs can adsorb a large amount of donor-specific antibodies. ■ Infections and lapses in immunosuppression can increase humoral immunity nonspecifically. ■ Increases in circulating antibodies accompanied by decreases in donor-specific antibodies could be indicative of deposition of donor-specific antibodies into the graft. ■ Decreases of circulating donor-specific antibodies can support an antibody-mediated rejection diagnosis, rather than rule it out. 11. A. Mother: DR1 DR15; father: DR3, DR7. Major points of discussion ■ Molecular methods using genomic DNA analysis are used to identify the HLA type of stem cell transplant recipients and donors. These methods include polymerase chain reaction (PCR) and DNA sequencing based methods. ■ Low-resolution HLA typing methods provide generic, antigen-level HLA typing data. Such methods use PCR-based techniques. ■ High-resolution HLA typing methods provide allele-level HLA typing data. Such methods use PCR- and DNA sequencing–based techniques. ■ The types of tissues most often used for HLA typing of stem cell recipients and donors are peripheral blood, umbilical cord, bone marrow, and buccal swabs. ■ HLA laboratories performing HLA typing for stem cell transplantation follow the guidelines of the National Bone Marrow Donor Registry.2,16 12. A. The results are invalid. Major points of discussion ■ Serum anti-HLA antibodies may occur as a result of sensitization to HLAs through the following: • Transfusion • Transplantation ■ Anti-HLA antibodies can be detected by the following: • CDC using a panel of HLA-typed cells • FC using HLA-coated beads • CDC or FC crossmatch using donor cells to detect anti-donor HLA antibodies. ■ Serum anti-HLA IgG antibodies are considered the most deleterious to the graft. The clinical significance of anti-lymphocytic IgM antibodies is not clearly understood. ■ To assess the cytotoxic activity of anti-lymphocytic antibodies, sera are tested in the presence and absence of DTT. DTT is a reduction agent, which destroys IgM structures. ■ A serum containing anti-donor IgG antibodies will cause a positive crossmatch when tested with or without prior treatment with DTT. ■ A serum containing anti-donor IgM antibodies, but not IgG antibodies, will cause a positive crossmatch when tested without DTT treatment and a negative crossmatch when tested after DTT treatment. 13. A. Types of T cells they stimulate. Major points of discussion ■ HLAs are the most polymorphic genes in the human genome. ■ HLA genes are co-dominantly expressed in each individual. ■ HLA proteins bind peptides derived from self and foreign protein antigens. HLA-peptide complexes are exposed on the cell surface of APCs and interact with the T-cell receptor of cytotoxic or helper T cells. ■ HLA class I proteins bind 9– to 12–amino acid long peptides from mostly intracellular proteins and interact with the T-cell receptor of CD8+ cytotoxic T cells. ■ HLA class II proteins bind 12– to 16–amino acid long peptides from mostly extracellular and membrane proteins and interact with the T-cell receptor of CD4+ helper T cells. ■ Recognition of foreign peptides presented in the context of self-HLA results in activation of T cells. 14. A. Co-dominance. Major points of discussion ■ Cross-reactivity is the reaction between an antibody and an antigen in which the antigen differs from the immunogen. ■ In a broader sense, cross-reactivity applies to other immune responses as well. For example, despite the high specificity of T-cell responses, some T-cell clones may react against more than a single antigen. ■ Cross-reactivity of immune cells has been documented in humans. For example, influenza virus–specific CD8+ T cells were shown to react against hepatitis virus antigen. ■ Cross-reactivity contributes to the vigorous response of T cells to allogeneic major MHC. T cells, which have been exposed to antigens, may cross-react with allogeneic cells. 15. A. Anti-donor HLA class I IgG. Major points of discussion ■ Anti-donor HLA antibodies are associated with increased risk of allograft rejection and graft loss. ■ To detect donor-specific antibodies in the serum of a transplant candidate, the patient’s serum is crossmatched with donor lymphocytes. A positive crossmatch is primarily due to anti-donor HLA antibodies. ■ CDC crossmatch is performed prior to transplantation to detect donor-specific cytotoxic antibodies. ■ FC crossmatch is performed prior to transplantation to assess the level of serum antibodies that bind to donor cells. This type of crossmatch is more sensitive than CDC crossmatch. ■ To reduce the titer of anti-HLA antibodies prior to transplantation, several desensitization protocols, such as plasmapheresis and treatment with IVIg or rituximab, are used. 16. A. Living related kidney transplantation in patients with high PRA. Major points of discussion ■ HLA typing is an important component of the immunologic evaluation of transplant candidates. ■ Low-resolution (antigen-level) HLA typing of recipient and donor is required for most solid organ transplants. ■ High-resolution (allele-level) HLA typing of recipient and donor is required for stem cell transplantation. ■ HLA matching is crucial for the success of stem cell transplantation. Lack of HLA matching may lead to graft failure and graft-versus-host disease, two major complications of stem cell transplantation. ■ PCR- and DNA sequencing–based methods are most often used for HLA typing. 17. A. FC crossmatch. Major points of discussion ■ Direct recognition of alloantigens results in a vigorous immune response, which leads to graft rejection. The ability of responding T cells to react against allogeneic cells can be assessed in vitro by measuring T-cell proliferation in a mixed lymphocyte culture. ■ Indirect recognition of allogeneic HLAs occurs through the interaction of T-cell receptors on the surface of host (responding) cells and peptides derived from allogeneic HLA proteins, presented in the context of self-HLA. ■ Indirect recognition of allogeneic HLAs results in activation of discrete subsets of T cells. However, as a result of continuous shedding of proteins from the graft, T-cell activation by the indirect recognition pathway is an ongoing process, which contributes to graft rejection. ■ Host CD4+ T helper cells activated by direct and/or indirect alloantigen recognition pathways promote activation and differentiation of antibody producing B cells. Production of anti-donor HLA antibodies may lead to irreversible rejection of the allograft by the mechanism known as complement-mediated cytotoxicity. 18. A. There is a high probability that the CDC crossmatch with an HLA-A2 donor will be positive. Major points of discussion ■ Anti-donor HLA antibodies are associated with increased risk of allograft rejection and graft loss. ■ Cytotoxic donor-specific antibodies may cause hyperacute or acute antibody-mediated rejection. ■ Sensitization to HLAs can occur through the following: • Blood transfusion • Transplantation ■ The techniques frequently used for the detection and identification of serum anti-HLA antibodies are: • CDC assay using a panel of HLA-typed cells. • FC assays using HLA-coated beads. They may be performed on a multiplex platform. • CDC or FC crossmatch with donor lymphocytes. ■ Virtual crossmatch determines whether a transplant candidate has anti-donor HLA antibodies and is at increased risk of rejection. Virtual crossmatch is positive if the HLA antibodies detected in the patient’s serum are specific for the donor HLA. This assessment facilitates the selection of the most suitable donor for an HLA-sensitized patient. 19. A. Dendritic cells enter the germinal centers through high endothelial venules. Major points of discussion ■ The lymph node acts a major communication hub between APCs and T cells. ■ Tissue-resident macrophages and dendritic cells enter the local draining lymph node through afferent lymphatics and present phagocytosed antigen to cognate T cells. ■ B cells, which encountered cognate antigen, form germinal centers in the lymph node where they proliferate, differentiate, class switch, and affinity mature their B-cell receptors (through somatic hypermutation). ■ The B-cell evolution through the germinal center is highly dependent on follicular dendritic cells (which sustain their activation with antigen) and T-cell help in the form of costimulation (CD40-CD40L, CD86-CD28 molecular cross-talk) and cytokines. 20. A. The patient is not at risk for EBV lymphoma. Major points of discussion ■ Allografts can transfer infection to a previously unexposed individual. ■ In the United States, over 90% of the adult population is EBV seropositive and 50% to 80% is cytomegalovirus (CMV) positive. Seroprevalence is age dependent. ■ Upon chronic immunosuppression, viral infections, which are kept under immunologic control in healthy individuals, can reactivate and become life threatening. ■ Posttransplant lymphoproliferative diseases (PTLDs) are dependent on the transplanted organ and level of immunosuppression. The highest incidence of PTLDs is observed in small bowel and multiple organ transplants (5% to 20%), followed by thoracic allografts (2% to 10%), and then by renal and liver transplants (1% to 5%).6,15 21. A. Proceed with the transplant because the auto-crossmatch establishes the threshold of a negative reaction. Major points of discussion ■ The relapse of the original autoimmune disease can happen after transplantation and contribute to the attrition of allografts. ■ However, patient and graft survival rates in transplanted autoimmune patients can approach those of nonautoimmune patients. For example, in kidney allograft recipients who displayed ANCAs before transplantation, the vasculitis relapse rate was 0.02 per patient-year and graft survival rates were 100% at 1 year, 93.4% at 5 years, and 67.4% at 10 years, in a Johns Hopkins study. ■ Kidney transplant is a safe and effective option for treating ESRD secondary to ANCA-associated vasculitis. ■ Autoimmune disease relapses are rare with current immunosuppression.7 22a. A. Donor 1 due to the allele mismatch with the patient, A*03:01 vs. A*03:04. 22b. A. Yes. Donor 3 is better than donor 2, but worse than donor 1. Major points of discussion ■ Allele-level HLA mismatches are sufficient to trigger the allorecognition of the entire host body by the transplanted immune system. ■ A study by Lee et al.22 has shown that high-resolution DNA (allele) matching for HLA-A, -B, -C, and -DRB1 (8/8 match) was the minimum level of matching associated with the highest survival. ■ A single mismatch detected by low- or high-resolution DNA testing at HLA-A, -B, -C, or –DRB1 (7/8 match) was associated with higher mortality (relative risk, 1.25; 95% CI, 1.13-1.38; P < .001) and 1-year survival of 43% compared with 52% for 8/8 matched pairs. ■ Single mismatches at HLA-B or HLA-C appear better tolerated than mismatches at HLA-A or HLA-DRB1. Mismatching at two or more loci compounded the risk. ■ Mismatching at HLA-DP or –DQ loci and donor factors other than HLA type were not associated with survival. In multivariate modeling, patient age, race, disease stage, and CMV status were as predictive of survival as donor HLA matching. ■ In a heterozygous recipient, an immune system generated from homozygous bone marrow donor (or a donor expressing only one allele) can recognize the mismatched allele as nonself and trigger GVHD.12 23. A. 75%. Major points of discussion ■ The HLA inheritance follows classic Mendelian inheritance principles for diploid organisms. ■ The HLA genes are located on chromosome 6p21 and are inherited together as a haplotype (except for rare crossover events within the locus). ■ Therefore, children are exactly haplo-identical to their parents, inheriting one HLA haplotypes (50% of their HLA makeup) from each parent. ■ The only exception is when one haplotype (or both) are represented in both parents. ■ Due to the highly polymorphic nature of the HLA genes, when parents share identical haplotypes, some level of shared ancestry can be inferred. 24. A. There are an unlimited amount of peptides that can be presented from a single protein. Major points of discussion ■ Unlike B cells, T cells recognize antigens only in the context of an MHC molecule. The recognition of a foreign antigen by T cells and its subsequent activation is predicated on the stable biochemical interaction between the TCR and the peptide-MHC complex on APCs. ■ Not all peptides can be loaded in all MHC molecules. Protein fragments must conform to a certain biochemical topology compatible with the MHC molecule. ■ MHC class I molecules (HLA-A, -B, -C) are ubiquitously and highly expressed on most tissues. They present intracellular proteins, which are derived from the organism’s own genome or from intracellular pathogens such as viruses. Only CD8+ T cells can be primed by presentation from MHC class I molecules. ■ MHC class II molecules (HLA-DR, -DP, -DQ) are expressed mostly by APCs and are loaded with proteins of extracellular origin following endocytosis. Only CD4+ T cells can be primed by presentation from MHC class II molecules. ■ The compartmentalization of intracellular-protein/class I/CD8 versus extracellular-protein/class II/CD4 is not absolute. Cross-presentation is the process by which some APCs allow extracellular antigen to be leaked out of the endosomes, loaded, and presented by MHC class I molecules. Thus, dendritic cells can prime both CD4+ and CD8+ T cells to phagocytose antigens.5,8 25. A. In infectious mononucleosis, the atypical lymphocytes are likely a large number of CD20+ B-cell blasts. Major points of discussion ■ Immunosuppression is nonspecific. It inhibits the immune response against the allograft, but it also reduces the immune surveillance against viral infections and tumors. ■ The majority (50% to 80%) of posttransplant lymphoproliferative disorder biopsy samples test positive for EBV.15 26. A. Cytokine receptors for interleukins (ILs)-2, -4, -7, -9, -15, and -21 signal through a common gamma chain–associated receptor activating JAK3 kinase. Major points of discussion ■ The effector function of CD4+ Th cells is largely dependent on the cytokine repertoire secreted on activation. ■ Th1 cells produce IFN-γ and enhance the killing activity of CD8+ cytotoxic T lymphocytes. ■ Th2 cells produce IL-4, -5, -6, -10, and -13 and enhance humoral responses from B cells. ■ Th17 effector cytokines are IL-17, -21, and -22, and they play an important role in antimicrobial immunity at epithelial/mucosal barriers. ■ T regulatory cells (some of which have been shown to act through IL-10 and TGF-β) inhibit the reactivity of other T cells to antigens. 27. A. Self-proteins cannot be processed into peptides. Major points of discussion ■ T- and B-cell clones that recognize self-peptides/epitopes do arise, but they are eliminated in the thymus or bone marrow, respectively, before they can enter the periphery. ■ Positive selection of nonreactive cells with functional T-cell receptors or B-cell receptors also takes place. Positive selection of self-cognate cells with regulatory function gives rise to “natural” T regulatory cell populations. ■ According to some estimates, over 90% of the T cells generated in the thymus are never released into the periphery. ■ Tissue-specific antigens are expressed in the thymus due to autoimmune regulator (AIRE) activation. ■ A key concept of alloreactivity is that adaptive immune cells are educated to be tolerant to self-HLAs during their development; however, their reactivity to foreign HLAs expressed by an allograft was never screened out. 28. A. Complement components. Major points of discussion ■ The disease association with HLA-DR4 suggests the activation of T cells to synovial antigens; however, depletion of B cells appears to be more effective in halting the disease. ■ A remarkable deceleration of disease progression is observed in many cases by blockade of the cytokine TNF-α. ■ Blockade of IL-1, IL-15, and IL-6 also has beneficial effects. ■ These manifestations suggest that both the B- and T-cell compartments are involved, with presentation of antigens by B cells to T cells by HLA-DR eliciting T-cell help and consequent production of rheumatoid factors and antibodies to citrullinated peptides. 29a. A. A non-HLA antibody. 29b. A. 29c. A. PRAs. Major points of discussion ■ A negative CDC crossmatch reaction allows a transplant to proceed with minimal risk of hyperacute rejection. The presensitization status of an allograft recipient may necessitate desensitization, induction therapies, and increased immunologic monitoring protocols by biopsy and serum antibody screening. ■ Antibody therapies designed to eliminate immune cell lineages (i.e., rituximab) interfere with cell-based assays (CDC and FC). ■ Humanized antibodies used for therapies can persist in circulation for months. ■ SPAs detect only antibodies against antigens coated on the solid phase (i.e., HLA-antigen–coated beads for the Luminex method). As a result, SPA testing is not susceptible to the iatrogenic effects of antibody therapies. 30. A. Narcolepsy. Major points of discussion ■ Multiple human diseases are associated with HLA alleles. ■ It is important to understand the difference between association and causation; HLA alleles associated with diseases may not always be pathogenic themselves. The culprit may be other (non-HLA) alleles specific to a human population, which also happens to be characterized by a high frequency of certain HLA alleles. ■ In the case of DQB1*06:02, it is one of the strongest disease associations of an HLA allele described. Some 90% to 100% of patients with definite cataplexy carry this allele. ■ The pathogenic role of HLA-DQB1*06:02 has not been fully elucidated, but as in all HLA-disease associations, an autoimmune mechanism is strongly suspected. ■ Recent studies describe a deficiency in hypocretin-secreting neurons in the hypothalamus of narcoleptic patients. HLA-DQB1*06:02 was shown to bind hypocretin with high affinity, and presumably present it to T cells, triggering an autoimmune response.3,17 31. A. Recipient peptides presented on donor MHC class II molecules to recipient T cells. ■ Recipient (self) peptides should not improve recipient TCR reaction to the donor MHC. ■ Most allograft parenchyma and stroma do not express MHC class II antigens and are unable to present exogenous (recipient) antigens. ■ Professional APCs of donor origin expressing MHC class II are not transferred in significant amounts to the recipient. ■ Recipient antigen endocytosed by donor endothelium (which can express MHC class II in inflammatory conditions) can be presented after the onset of inflammation. B. Complementarity-determining regions (CDRs) of recipient TCR stably binding the polymorphic domains of the donor MHC. Major points of discussion ■ There are two modalities of allorecognition by T cells: the direct and indirect pathways. ■ The direct allorecognition pathway is driven by the stable binding of some of the TCRs of the recipient’s T-cell clones to the donor MHC. This interaction with self-MHC is screened out in the thymus, and no strongly self-reactive TCRs are allowed to exit into the periphery. The interaction with donor MHC was never screened for in the same manner. ■ The indirect allorecognition pathway is the transplantation equivalent of T cells’ immunity to any foreign antigen. Exogenous (donor) antigens are phagocytosed by (recipient) APCs and presented to (recipient) T cells in the context of self-MHC. ■ The direct allorecognition is the dominant process in HLA-mismatched transplantation. ■ The indirect recognition is evidenced by rejection in transplantation between HLA-matched siblings (not identical twins). In this scenario, the HLA molecules are identical and cannot generate direct allorecognition, but they can present polymorphic molecules by means of the indirect pathway. ■ Siblings can inherit the same maternal and paternal chromosome 6 (or HLA region only) but be genetically different at any other locus due to the stochastic nature of chromosome segregation during gametogenesis.13,14 32. A. Cyclosporin A. Major points of discussion ■ If the patient is presensitized and has antibodies against the donor’s alloantigens (most commonly, HLAs) in circulation, the serum will opsonize and fix complement on the donor cells, killing them. ■ A positive CDC crossmatch is a strong contraindication for transplantation. Presensitized transplant recipients who have a positive CDC crossmatch with their donors may hyperacutely reject their allograft. ■ A T- and B-cell–positive crossmatch indicates the presence of anti-HLA class I antibodies (since HLA class I is ubiquitously expressed on human tissues and cells). ■ A B-cell–positive crossmatch with a negative T-cell crossmatch indicates the presence of anti-HLA class II antibodies since HLA class II is expressed only on APCs such as B cells. ■ Anti-thymocyte globulin and basiliximab are antibodies against T-cell antigens. Rituximab is a humanized anti-CD20 antibody (a B-cell lineage–specific marker). All of these antibodies can give false-positive T- or B-cell CDC crossmatch results. 33. A. HLA-A and HLA-DP. Major points of discussion ■ Recombination between HLA genes is relatively rare since all HLA genes are contained together on chromosome 6p21.31. ■ The HLA-DR and –DQ genes are in linkage disequilibrium, such that the DQ typing could be predicted fairly reliably from the DR typing. 34. A. Drug toxicity. Major points of discussion ■ In cases of organ transplantation from HLA-identical siblings, direct allorecognition cannot occur, since the MHC molecules of the donor and recipient are identical. ■ Nevertheless, rejection of allografts from HLA-identical siblings does occur due to indirect allorecognition. ■ Indirect allorecognition is the process by which an allopeptide is presented in the context of self-MHC molecules and elicits the activation of cognate T-cell clones. ■ Siblings can inherit the same maternal and paternal chromosome 6 (or HLA region only) haplotypes but still be genetically different at any other polymorphic locus due to the stochastic nature of chromosome segregation during gametogenesis. ■ Importantly, indirect allorecognition can occur in HLA-identical siblings but not in monozygotic twins (who are 100% genetically identical). ■ In HLA-mismatched transplantation, direct and indirect allorecognition can occur in parallel, and the peptides providing the basis of indirect allorecognition can be derived from the mismatched donor MHC molecules.13,14 35. A. It is much better than other solid organs because the lung is an immune-privileged site. Major points of discussion ■ Lung and small bowel allograft survival is significantly worse than that for other solid organs. ■ Lung allograft survival is affected by the complexity of the surgical procedure, area of exposure to the external environment, and size-matching requirements. ■ Infections represent a major morbidity factor in transplantation. Some, such as bacterial pneumonia, are particular to lung transplantation, whereas others are the result of chronic immunosuppression and are common to many types of transplantation (i.e., CMV infections). Cystic fibrosis, one of the major pathologies addressed by lung transplantation, is notorious for its association with persistent airway infections. ■ BOS is a lung-specific fibroproliferative reaction in the small airway lumens, leading to allograft dysfunction. BOS is caused by alloimmune, autoimmune, and nonimmunologic factors and has a major impact on lung allograft survival.
Immunology, Immunogenetics
HLA-A1/A26
HLA-B42/B16
HLA-DR3/DR4
As a standard procedure, a recipient blood sample is retyped at his current institution with the following results:
HLA-A1/A26
HLA-B42/B38
HLA-DR17/DR4
Which one of the following would account for this discrepancy?
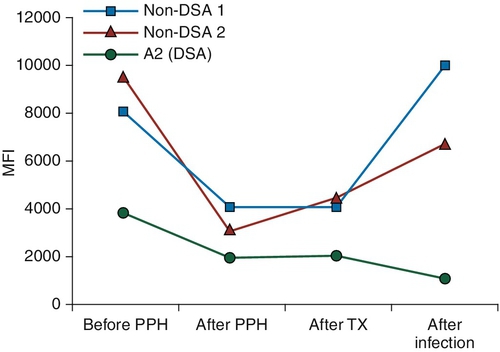
Patient: HLA-A2, -A3, -B7, -BX, -DR7, -DR11
Donor: HLA-A2, -A3, -B7, -B27, -DR7, -DR11
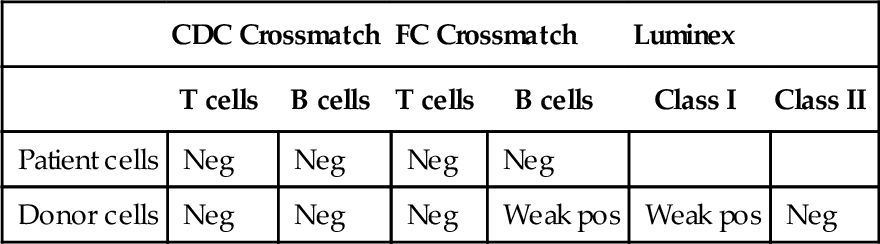
The patient develops de novo alloantibodies detected on routine serum monitoring on 50% of a panel of cells (PRA ~ 50%). SPAs using individual HLA-coated beads show antibodies reacting with over 20 HLA-B specificities, including HLA-B27. The serum also shows positive reactions against HLA-A23, -24, -25, and -32. The patient’s medical history records no other sensitizing events other than the transplant. Which one of the following is the best explanation?
Serum
T-Cell Crossmatch Result
B-Cell Crossmatch Result
Negative control serum
Neg
Neg
Positive control serum
Pos
Pos
Patient serum, untreated
Neg
Neg
Patient serum, dithiothreitol treated
Pos
Pos
Patient
A*02:01, A*03:01
Donor 1
A*02:01, A*03:04
Donor 2
A*02:01, A*01:01
Rationale: This appears to be a case of HLA class I and class II sensitization with an anti-HLA class II donor-specific antibody, which also fixes complement (CDC crossmatch B cells +). An IgG anti-HLA class I antibody is also detected but is either of insufficient titer, affinity, or wrong isotype subtype to fix complement.
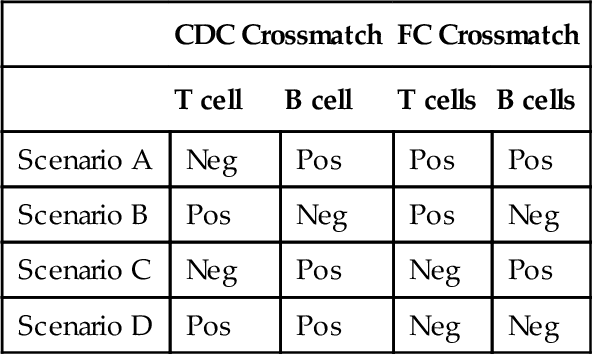
B. Scenario B.
Rationale: This appears to be a non-HLA antibody to an antigen present on T cells and not B cells. An iatrogenic effect of monoclonal antibody therapy such as muromonab-CD3 (trade name Orthoclone OKT3) or anti-thymocyte globulin (ATG) should be suspected.
C. Scenario C.
Rationale: The results suggest a complement fixing (CDC +) anti-HLA class II antibody (B cells +), without any anti-HLA class I antibodies.
D. Scenario D.
Rationale: This result is due to one or more IgM isotype antibody(ies), which is(are) not detectable by FC crossmatch but can fix complement. Despite their ability to fix complement, IgM antibodies do not present a grave danger to allografts.
Rationale: Unambiguous result: HLA class I and class II sensitization, with a class II donor-specific antibody that also fixes complement (CDC crossmatch B cell +).
B. Scenario B.
Rationale: Ambiguous result: It could be a non-HLA antibody present on T cells and not B cells or an iatrogenic effect of anti–T-cell monoclonal antibody therapy such as muromonab-CD3 or ATG. Further testing by SPAs with single antigen beads or enzyme-linked immunosorbent assay (ELISA) may elucidate this.
C. Scenario C.
Rationale: Unambiguous result: A complement fixing (CDC +) anti-HLA class II antibody (B cells +), with no anti-HLA class I antibodies detected.
D. Scenario D.
Rationale: Unambiguous result: This result is due to an IgM isotype antibody(ies), which is(are) not detectable by FC crossmatch but can fix complement.
B. Scenario B.
C. Scenario C.
Rationale: A positive FC crossmatch implies an IgG antibody.
D. Scenario D.
Rationale: This result is due to one or more IgM isotype antibody(ies), which is(are) not detectable by FC crossmatch but can fix complement. Standard FC crossmatch methodology is designed to detect only IgG antibodies.
Rationale: Following immune reactions, it is thought that some antigen is always retained by follicular dendritic cells in the lymph nodes maintaining long-term memory responses. Antigen storage and increases in circulating antibodies can occur regardless of the “thoroughness” of the nephrectomy.
B. Anti-donor HLA antibodies continue to be produced after nephrectomy; however, the graft no longer adsorbs them.
Rationale: Anti-donor HLA antibodies are secreted by plasma cells. These plasma cells can be long lived and are not vulnerable to rituximab treatment due to downregulation of surface CD20 antigen. After nephrectomy, plasma cells continue to produce antibodies, but the graft no longer acts as a sink for the circulating antibodies.
C. The readings are an artifact of the rituximab treatment, which can persist in circulation many months after the treatment.
Rationale: Whereas rituximab can interfere with a B-cell crossmatch, SPAs use beads coated only with HLAs. Therefore, antibodies against antigens other than HLA cannot be detected in SPAs.
D. The histocompatibility lab switched reagent lots, and the new reagents are much better than the old ones at detecting the antibodies against the specific allele of the donor in this case.
Rationale: Clinical labs must test reagents for efficacy and consistency from lot to lot. Although not inconceivable, this is an extremely remote possibility.
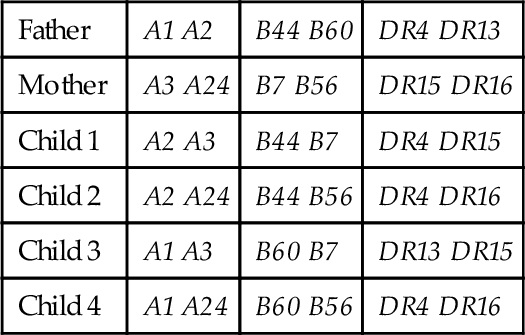
B. Child 2.
C. Child 3.
D. Child 4.
Rationale: If the parental haplotypes are designated as:
a: A2 B44 DR4
b: A1 B60 DR13
c: A3 B7 DR15
d: A24 B56 DR16
The family haplotype makeup is:
Father a/b
Mother c/d
Child 1 a/c
Child 2 a/d
Child 3 b/c
Child 4: a + b/d
Rationale: HLA nomenclature was initially assigned according to common alloantibodies that recognized them. It was later recognized that some antibodies detected multiple antigens and the nomenclature was redefined by renaming the old antigen into the two or more newly discovered “splits.” For example, HLA-B16 split into B38 and B39 and HLA-DR3 became DR17 and DR18.
B. Egregious errors of the previous histocompatibility lab.
C. Egregious errors of the current histocompatibility lab.
Rationale: The first and second typings are actually concordant.
D. The donor’s HLAs are detected in the patient’s blood.
Rationale: A kidney does not shed sufficient amounts of antigens or cells into the circulation of a recipient to interfere with HLA typing.
Rationale: The number of mismatches does not determine the amount of donor-specific antibodies in circulation.
B. Number of previous transplants.
Rationale: The number of previous transplants does not determine the amount of donor-specific antibodies in circulation.
C. The PRA percentage.
Rationale: Even in a patient with high PRA (i.e., >80%), the antibodies may not be donor specific.
D. Titer of donor-specific antibodies.
Rationale: High titers of donor-specific antibodies can result in hyperacute rejection. Lowering these titers to levels at which the patient no longer has a positive crossmatch with the donor is paramount before proceeding with the transplant.
Rationale: Whereas the A2 serotype is indeed present in 40% of many human populations, individual HLA alleles (i.e., A*02:04) are present in smaller fractions and, hence, would not justify a 50% PRA. Cross-reactivity of antibodies between closely related HLAs is common, but they usually involve a few antigens, not dozens.
B. The patient produced multiple anti-HLA antibodies against mismatched, nontyped loci (HLA-C, -DQ, -DP), which cross-react with multiple HLA-A and -B alleles.
Rationale: Two facts make this scenario implausible: (1) If anti-HLA-C/DQ/DP reactions occurred, the SPA failed to detect them, and (2) the cross-reactivity of antibodies across loci is simply not as extensive as it would need to be for this explanation to be true.
C. The patient produced antibodies against the β-2 microglobulin chain of HLA class I expressed by half of the cell panel and used by all the HLA alleles identified by SPAs.
Rationale: The β-2 microglobulin is a nonpolymorphic chain in all HLA class I molecules. It is a self-antigen for the recipient, so the patient should not be able to make antibodies against it. If such an autoantibody should arise, it would test positive on 100% of the panel.
D. The patient produced a single antibody against the epitope Bw4 present in HLA-B27 and many HLA-A and -B alleles.
Rationale: Bw4 is a “public epitope” composed of four residues. It appears on many HLA-B and some HLA-A alleles.
Rationale: This may be the best pairing. Some evidence suggests that antigens make it across the placental barrier, and children establish central tolerance to maternal antigens providing the basis for better transplant outcomes than other parent-child pairings.
B. Father donates to child.
C. Child donates to mother.
Rationale: Fetal antigens (of paternal genetic origin) can be transferred to the mother during the pregnancy, and the mother’s immune system can form antibodies against them. This can represent a presensitization event for a child-to-mother organ transplant, and the rejection risk is higher than in the other donor-recipient pairings shown here.
D. Child donates to father.
Rationale for B and D: The donor and recipient share one half of their genetic material, including one half of all the HLAs (all loci). This scenario does not have any additional benefits or detractions like the mother-child scenarios.
Rationale: Auto-crossmatch is defined as the patient’s serum reactivity with his or her own cells.
B. Direct crossmatch.
Rationale: Direct crossmatch is defined as the patient’s serum reactivity with his or her donor’s cells.
C. Virtual crossmatch.
Rationale: Crossmatch results can be accurately predicted when the patient’s antibody specificities have been identified using recombinant single HLA bead technologies and the potential donor HLA type is known.
D. Flow crossmatch.
Rationale: Flow crossmatch analyzes the reactivity of patient’s serum alloantibodies with a donor’s cells by FC.
Rationale: Sepsis can in fact cause thrombus formation; however, no mention is made of the patient being septic.
B. Graft dysfunction caused by a humoral response against the pathogen, which cross-reacts with non-HLA heart antigens.
Rationale: β-Hemolytic streptococci, but not S. pneumoniae, can induce anti-myosin antibodies by molecular mimicry.
C. Antibody-mediated rejection caused by complement-fixing non–donor-specific antibodies that cross-react with donor HLA alleles.
Rationale: Cross-reactivity of anti-HLA antibodies with closely related HLA alleles has been long documented. Antibodies classified as non–donor-specific remained in circulation at high levels throughout this patient’s history. However, they caused neither a positive crossmatch nor hyperacute/acute rejection, so they are not the likely cause of humoral rejection.
D. Antibody-mediated rejection caused by the anti–HLA-A2 antibody, which increased along with other anti-HLA antibodies as a consequence of the infection.
Rationale: Humoral immunity including anti–HLA-A2 antibody production increased as a consequence of the infection. Solid organs can absorb a large amount of antibodies and the levels of circulating anti-A2 antibody misleadingly appear to decrease during rejection.
B. Mother: DR1 DR3; father: DR4, DR7.
C. Mother: DR1 DRX; father: DR1, DR4.
Rationale: This family will benefit most from allele-level typing for the identification of an HLA-identical sibling. Allele-level typing of the parents’ HLA genes will identify the subtypes of the DR1 gene carried by the mother and father. Allele-level typing of the children will facilitate the identification of parental HLA haplotypes and recognition of HLA-identical siblings.
D. Mother: DR11 DR4; father: DR3, DR7.
Rationale for A, B, and D: HLA-identical siblings can be identified with low-resolution (antigen-level) HLA typing in this family.
Rationale: This pattern of reactivity can occur in donor-recipient crossmatching.
B. The serum contains a donor-specific IgG antibody and a blocking IgM antibody.
Rationale: A blocking IgM antibody may interfere with donor-specific IgG antibody, resulting in a negative crossmatch. This effect is abolished on treatment of the serum with a reducing agent, such as DTT.
C. The serum contains a donor-specific IgM antibody and a blocking IgG antibody.
Rationale: A donor-specific IgM antibody typically causes a positive crossmatch with the untreated serum and a negative crossmatch with the DTT-treated serum.
D. The serum contains a donor-specific IgA antibody.
Rationale: Cytotoxic anti-lymphocyte IgA antibodies are extremely rare. In addition, the pattern of serum reactivity is consistent with the presence of a blocking IgM antibody.
Rationale: HLA class I and class II molecules stimulate CD8+ and CD4+ T lymphocytes, respectively.
B. Types of cells that express them.
Rationale: Most cells express HLA class I antigens. However, expression of HLA class II is restricted to antigen presenting cells, such as dendritic cells, monocytes, macrophages and B lymphocytes.
C. The chromosome on which they are located.
Rationale: Both HLA class I and class II genes are located on human chromosome 6.
D. Types of antigens they present.
Rationale: HLA class I predominantly presents peptides derived from intracellular proteins, whereas HLA class II presents mostly peptides derived from extracellular and membrane proteins.
E. Types of cells that contain a β-2 microglobulin subunit.
Rationale: Only HLA class I proteins contain a β-2 microglobulin subunit.
Rationale: Co-dominance is the relationship between two alleles from a single locus, which contribute equally to the phenotype.
B. Cross-reactivity.
Rationale: See Major Points of Discussion.
C. Memory response.
Rationale: Memory response is the enhanced immune response that occurs on reexposure to antigen.
D. Mixed lymphocyte reaction.
Rationale: Mixed lymphocyte reaction is an in vitro assay that measures T-cell proliferation triggered by HLA-mismatched cells.
E. PRA.
Rationale: PRA is a measure of anti-human antibodies present in the patient serum. PRA estimates the percentage of individuals in a population who will be excluded as transplant donors for that patient because of a positive crossmatch.
B. Anti-donor HLA class I and class II IgG.
C. Anti-donor non-HLA IgG.
Rationale: These types of antibodies may cause a positive FC crossmatch. However, a positive FC crossmatch is most frequently caused by anti-donor HLA class I and/or class II antibodies.
D. Anti-donor lymphocyte IgG.
Rationale for A, B, and D: FC crossmatch performed with donor lymphocytes detects anti-donor HLA class I and class II antibodies as well as non-HLA antibodies, which bind to donor cells. In addition, FC crossmatch also detects anti-donor non-HLA antibodies.
E. Cytotoxic anti-donor HLA IgG and IgM.
Rationale: This test does not assess cytotoxicity.
B. Liver transplantation.
Rationale: High-resolution HLA class I and class II typing is not required in solid organ transplantation. Many liver transplant programs do not require HLA class I and class II typing at any level of resolution. Anti-HLA allele-specific antibodies are rare.
C. Stem cell transplantation: unrelated donor.
D. Stem cell transplantation: two haplotype–matched related donor.
Rationale: High-resolution HLA class I and class II typing is required in stem cell transplantation. In the setting of unrelated donor transplantation, high-resolution typing is particularly important because of the higher probability of an allele-level mismatch between the donor and recipient. A single mismatch detected with either low-resolution or high-resolution DNA testing is associated with higher mortality. The risk is compounded with two or more mismatches.
E. ABO-incompatible renal transplantation.
Rationale: High-resolution HLA class I and class II typing will not add useful information to that provided by low-resolution typing in the setting of ABO-incompatible transplantation.
Rationale: This test detects the recipient’s antibodies that bind to donor lymphocytes.
B. CDC crossmatch.
Rationale: This test detects recipient’s antibodies that are able to kill donor lymphocytes.
C. HLA typing using sequence-specific oligonucleotides (SSOs).
Rationale: HLA typing using SSOs involves DNA isolation and SSO hybridization. It does not involve T-lymphocyte recognition of alloantigens.
D. Mixed lymphocyte culture.
Rationale: This test measures proliferation of T lymphocytes in response to direct recognition of cell surface alloantigens, in particular allogeneic HLA.
E. Mitogen T-cell stimulation.
Rationale: This test measures proliferation of T lymphocytes in response to a mitogen and does not involve recognition of alloantigens.
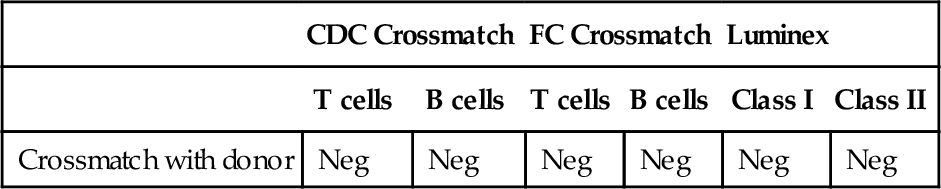
Rationale: It is unlikely that the CDC crossmatch will be positive because the patient’s serum does not contain detectable cytotoxic antibodies.
B. There is an increased risk of acute antibody-mediated rejection if the patient received an HLA-A2 transplant.
Rationale: Patients with preformed donor-specific antibodies are at increased risk of acute antibody-mediated rejection compared with patients without donor-specific antibodies.
C. There is a high risk of hyperacute rejection if the patient received an HLA-A2 transplant.
Rationale: There is no evidence that anti-donor HLA antibodies detected exclusively by solid-phase immunoassay cause hyperacute rejection.
D. Transplantation with an HLA-A2 graft is contraindicated even if the CDC crossmatch is negative.
Rationale: The presence of a donor-specific antibody that is undetectable by CDC is not considered a contraindication for transplantation.
E. The results of the CDC and solid-phase immunoassays are discordant and, therefore, they are invalid.
Rationale: CDC and solid-phase immunoassays have different sensitivity levels in detecting anti-HLA antibodies. Solid-phase immunoassays are more sensitive than CDC-based assays and may detect low-titer/low-strength antibodies.
Rationale: Lymphocytes enter lymph nodes from the blood through high endothelial venules, whereas most dendritic cells enter the lymph node from the tissue through the lymphatics into the cortical sinus.
B. Long-lived plasma cells reside primarily in the germinal center and paracortical areas.
Rationale: Long-lived plasma cells reside primarily in the bone marrow.
C. Antigen-independent proliferation of B cells occurs in lymph nodes.
Rationale: Antigen-independent stages of B cell development occur in the bone marrow. Antigen-dependent proliferation occurs in the secondary lymphoid organs (lymph nodes and spleen).
D. Activated T cells are absent from germinal centers.
Rationale: Activated T cells and B cells are the mutually dependent driving force of the germinal center response. B-cell presentation of antigen to T cells activates responding T cells, which in turn drive B-cell activation.
E. Affinity maturation of B cells occurs in the germinal center.
Rationale: B-cell somatic hypermutation and class switching require CD40 engagement by CD40L-expressing helper T cells in the germinal center. Defective CD40 signaling is responsible for the hyper IgM syndrome.
Rationale: Although there is evidence of B-cell polyclonality with mixed κ/λ light chain expression, clonal expansion is not excluded and the CD43 dim subset should be assessed for evidence of clonality.
B. EBV reactivation in immunocompromised patients is due to humoral insufficiency and can be overcome with IVIg treatment.
Rationale: Defective cell-mediated immunity, in particular reduced activity of EBV-specific effector memory CD8 T-cell activity, is likely to be responsible for this failure of immune surveillance.
C. B-cell proliferation in EBV lymphoproliferative disorders is due to B-cell receptor–mediated B-cell recognition of EBV antigens.
Rationale: EBV-related posttransplant lymphoproliferative disorder is due to the activation of EBV-infected B cells by EBV-encoded oncoproteins.
D. The receptor for EBV viral entry on B cell is the complement receptor CD21.
Rationale: See Major Points of Discussion.
E. EBV lymphoma is less commonly seen in pediatric transplants since posttransplant lymphoproliferative disorder is due to EBV reactivation from prior infection.
Rationale: Primary EBV infection in the posttransplant period is a major cause of EBV posttransplant lymphoproliferative disorder in the pediatric population in which 50% or more of recipients may not have been previously exposed to EBV.
Rationale: A negative control (serum from a nonsensitized donor) is included in the flow crossmatch test, and it serves to establish the negative threshold for the crossmatch reaction.
B. Rule out this donor because the crossmatch is positive regardless of the auto-crossmatch results.
C. Do not transplant. These are autoantibodies (auto-crossmatch positive) that recognize the same antigen on donor cells and, therefore, will destroy the transplanted kidney as well.
D. Perform SPA testing to determine whether the antibodies are anti-HLA antibodies, and if yes, whether they are donor specific.
Rationale: The SLE diagnosis and the positive auto-crossmatch suggest that an autoantibody might be present. Patients with autoantibodies are clinically manageable and fare quite well after transplantation, so this is not an absolute contraindication to transplantation. However, it is certain that the patient has antibodies in circulation and the presence of a donor-specific anti-HLA antibodies must be ruled out.
Rationale: Allele mismatches are dangerous in bone marrow transplantation, reducing the 1-year survival by about 10% for every mismatch.
B. Donor 2 due to the antigen mismatch with the patient, A*03:01 vs. A*01:01.
Rationale: Antigen mismatches are dangerous in bone marrow transplantation and can result in graft versus host disease or poor engraftment, reducing the 1-year survival by about 10% for every mismatch.
C. No difference between donors 1 and 2 regarding the impact of mismatch on graft survival.
Rationale: Allele- and antigen-level mismatches appear equally deleterious in terms of the impact on graft survival.
D. Depends on where the mismatched amino acid(s) lie in the HLA structure.
Rationale: Most polymorphic sites in HLA molecules lie in the α helices flanking the peptide groove and interact with the T-cell receptor’s complementarity-determining regions (CDRs).
Rationale: All three donors would be considered to have one mismatch at the HLA-A locus to the recipient.
B. No. Donor 3 has an exotic allele, which is highly likely to elicit allorecognition and trigger graft-versus-host disease (GVHD).
Rationale: All allele groups designated by identical first numbers (i.e., all A*02 alleles) are similar enough that they are hardly distinguishable by serology methods. In this case, A*02:305 N differs by a single nucleotide deletion from A*02:01:01; however, this causes it to be a null allele, which yields no protein.
C. Yes. Donor 3 has a perfectly matched allele A*02:01 and a null allele A*02:305 N, which does not contribute to the allorecognition.
D. No. Homozygosity or single-allele expression at one locus in the donor may trigger GVHD.
Rationale: The donor cells would recognize the host A*03:01 as allogeneic/nonself, and this recognition could be the basis of a GVHD reaction. Donor homozygosity at a locus counts as a mismatch in stem cell transplantation. Therefore, donor 3 is not a better choice than either donor 1 or 2.
B. 50%.
C. 25%.
D. 16.7%.
E. 0%.
Rationale: Since a child inherits one HLA haplotype from each parent, a child will typically be 50% matched to each parent. Exceptions in which children are 100% HLA matched to a parent can occur when the parents share HLA haplotypes—that is, closely related parents, isolated communities with a strong founder effect, and so on.
Rationale: Peptides bind to HLA molecules through anchor residues to specific HLA molecules. Each HLA molecule has specific anchor residue requirements for binding that limit the potential peptides that can be presented from even large proteins. Although each HLA molecule can present only a limited number of peptides, HLA diversity within the human population is likely to be important evolutionarily to enable a broad capacity of antigen-presenting structures to broaden responsiveness to microbes across the population.
B. HLA haplotypes can contribute to immune responsiveness to a specific virus or vaccine.
Rationale: Since HLA molecules vary according to their ability to bind specific amino acid sequences, some HLA molecules are better than others in presenting specific viral peptides to T cells. For example, specific HLA alleles correlate with hepatitis B vaccine responsiveness as a result of their differing ability to present hepatitis B virus surface antigen peptides to helper T cells.
C. Only extracellular antigens can be presented on HLA class II molecules.
Rationale: While the exogenous pathway is the dominant pathway for HLA class II loading by peptides in the endosomal system, intracellular antigens can be presented as well, including by means of autophagy.
D. Only intracellular antigens can be presented on HLA class I molecules.
Rationale: Although the endogenous pathway is the dominant pathway for HLA class I loading, the cross-presentation pathway also allows extracellular antigens to be presented.
E. Only the amino acid sequence determines the immunogenicity of peptides loaded onto HLA molecules.
Rationale: Classic HLA molecules can present modified peptides. Although binding to the HLA molecule strictly requires anchor amino acid interactions with the HLA groove, the polypeptide can have linked glycosylations or other modifications that protrude from the cleft and interact with T-cell receptor contacts, thus contributing to the immunologic “epitope.” Moreover, nonclassic HLA molecules can present glycolipids as, for instance, cell wall constituents of tuberculosis.
Rationale: Primary EBV infection is associated with a reactive lymphocytosis that includes circulating EBV-specific CD8+ T cells.
B. In infectious mononucleosis, the atypical lymphocytes are likely a large number of activated CD8+ T cells.
Rationale: See Major Points of Discussion.
C. B-cell leukemia can be excluded if the atypical cells are surface B-cell receptor negative.
Rationale: Pre–B-cell leukemia staining will identify cytoplasmic μ chains, which are identified only by staining of permeabilized cells.
D. EBV-positive serology is seen in around 50% of adults.
Rationale: In the adult population, EBV-positive serology is more than 90%.
E. Stable integration of the EBV genome into chromosomal DNA of infected B cells is required for latency.
Rationale: The EBV genome can exist in infected B cells either episomally or integrated into the chromosome.
Rationale: Genetic deficiency in the common gamma chain results in X-linked severe combined immunodeficiency, including loss of B, T, and natural killer (NK) cells caused by the loss of IL-2, -7, and -15. Anti-inflammatory agents targeting the JAK3 tyrosine kinase are in clinical development to treat autoimmunity and to prevent/treat solid organ transplant rejection.
B. T-cell production of interferon (IFN)-γ, IL-12, and IL-17 augments the cellular immune response.
Rationale: These cytokines are potent proinflammatory agents that boost cellular immunity, but APCs are the primary sources of IL-12. IFN-γ is made by T helper (Th)1 cells and NK cells, and IL-17 is made by Th17 cells.
C. Cyclosporine blocks T-cell receptor signaling via inhibition of the tyrosine kinase nuclear factor of activated T cells (NFAT).
Rationale: NFAT is a transcription factor (not a kinase) activated on T-cell receptor activation of T cells and drives the transcription of T-cell–derived cytokines, including IL-2. Cyclosporine prevents the dephosphorylation of NFAT by binding to cyclophilin.
D. Toll-like receptors recognize only microbial constituents and activate innate immune cells through necrosis factor (NF)- κB.
Rationale: Toll-like receptors (TLRs) certainly recognize microbial constituents and signal through the NF-κB transcription factor. However, it has become clear that TLRs can recognize self/endogenous ligands. For instance, endosomal TLRs TLR7 and TLR9 are activated by CpG motifs in chromatin and ribonuclear proteins, respectively, and HMGB-1 is a ligand for TLR4. Thus, innate activation by self- and graft-derived ligands could contribute to autoimmunity and graft rejection.
E. Regulatory T cells have the capacity to induce tolerance by killing self-reactive effector T cells.
Rationale: Regulatory T cells are not thought to have cytolytic capacity. Regulatory T cells inhibit APCs and responding T cells through the production of anti-inflammatory cytokines IL-10 and transforming growth factor (TGF)-β and through contact-dependent mechanisms.
B. Peptides from self-proteins cannot bind to HLA class I molecules.
Rationale: HLA class I molecules primarily present intracellular self-peptides.
C. Peptides from self-proteins cannot bind to HLA class II molecules.
Rationale: Phagocytes can take up and present necrotic or apoptotic cells and material, which include self-antigens.
D. Lymphocytes reactive to self-proteins are inactivated by deletion, anergy, or receptor editing.
Rationale: Negative selection generally ensures that a lymphocyte expressing a receptor reactive to a self-protein is eliminated by deletion, anergy induction, or receptor editing in the case of an autoreactive B cell.
E. Developing lymphocytes cannot rearrange V genes required to produce a receptor for self-proteins.
Rationale: Such rearrangements can happen, but self-reactive cells are negatively selected out.
B. Rheumatoid factor.
C. Tumor necrosis factor (TNF) and IL-1.
D. IL-4 and IL-10.
Rationale: Patients with rheumatoid arthritis have elevated levels of proinflammatory cytokines TNF and IL-1 in their joints, which play a role in the pain, swelling, stiffness, and other symptoms associated with this disease.
Rationale: Luminex SPAs can detect only anti-HLA antibodies.
B. An HLA class I antibody.
Rationale: In order of sensitivity, the methods listed are CDC < FC crossmatch < SPA/Luminex. A low-titer anti-HLA class I antibody would explain the Luminex/SPA reaction and the partial flow crossmatch–positive reaction on B cells (B cells express HLA class I and class II).
C. An HLA class II antibody.
Rationale: An anti-HLA class II antibody is inconsistent with the SPA results, which show only an anti-HLA class I antibody.
D. An autoantibody.
Rationale: Autoantibodies are not directed against HLAs, which are the only antigens coated on Luminex beads (the one exception is MHC class I polypeptide related sequence A [MICA] antigens).
E. Major histocompatibility complex (MHC) class I polypeptide related sequence A (MICA) antigen antibody.
Rationale: MICA antigens are distinguishable from HLA class I and class II on Luminex beads. They do not give positive reactions on B cells, as they are mostly expressed on stressed endothelial cells.
Rationale: Rituximab treatment affects some of the testing methodology.
B.
Rationale: Rituximab would give positive crossmatches by FC and CDC. It would not affect SPA results.
C.
Rationale: If the anti-HLA class I antibody detected pretransplantation is a donor-specific antibody and is induced by the allograft despite induction therapy, it would likely be first observed by the most sensitive method, which is the SPA/Luminex. It would likely also take longer than 1 week to generate positive crossmatches.
D.
Rationale: Rituximab treatment would not be detected by SPA/Luminex because the rituximab target, CD20, is not represented on the Luminex beads. CDC crossmatch, however, should have been positive on B cells.
Rationale: PRA is a CDC-based method and the CDC crossmatch does not appear to detect this antibody. The PRA would not offer additional information.
B. Luminex/SPA with single antigen beads.
Rationale: Luminex/SPA with single antigen beads uses beads coated with individual antigens and can determine the HLA specificity of the anti–class I antibody present in this serum.
C. T- and B-cell CDC crossmatch with anti-human globulin.
Rationale: Anti-human globulin is used to enhance the sensitivity of the CDC crossmatch against T-cell targets. All B cells express B-cell receptors, which are essentially cell surface antibodies. The use of anti-human globulin on B cells would give a certain false-positive reaction.
D. High-resolution typing of the recipient and donor.
Rationale: Unlike in stem cell transplantation, solid organ recipients and donors are not HLA matched at the allele level. Such typing would give no additional information about the antibody present in this serum.
Rationale: Narcolepsy has one of the tightest known associations to an HLA allele. DQB1*06:02 constitutes the major HLA susceptibility allele across all ethnic groups.
B. Celiac disease.
Rationale: Celiac disease is associated with HLA-DQ2/DQ8.
C. Ankylosing spondylitis.
Rationale: Ankylosing spondylitis is associated with HLA-B27.
D. Behçet’s disease.
Rationale: Behçet’s disease is associated with HLA-B51.
Rationale: This process cannot be the basis of strong recognition between recipient T and donor cells because of the following:
Rationale: The hypervariable CDRs of recipient TCR, which stably bind the polymorphic grooves of self MHC, have been negatively selected against in the recipient thymus. However, no such selection happened for the recipient CDRs/donor MHC pairing. This interaction can be very strong by itself and can only be made worse by presentation of donor (exogenous) peptides in the MHC groove.
C. Stable binding of CD4 co-receptor molecules to nonself MHC class II alleles.
D. Stable binding of CD8 co-receptor molecules to nonself MHC class I alleles.
E. Stable binding of both CD4 co-receptor molecules to nonself MHC class II alleles and stable binding of CD8 co-receptor molecules to nonself MHC class I alleles.
Rationale: CD8 and CD4 molecules bind to nonpolymorphic domains of MHC class I/II and, therefore, the strength of these interactions is not dependent on the MHC allele. If this were the case, all T cells would be alloreactive in MHC-mismatched transplantation. The alloresponse in transplantation is oligoclonal.
Rationale: Cyclosporin A binds to the cytosolic protein cyclophilin in lymphocytes. This complex inhibits calcineurin, which is responsible for activating the transcription of IL-2. It is not an antibody, and it does not interfere with antibody binding to cells. Therefore, it does affect FC crossmatch results.
B. Rituximab.
Rationale: Rituximab is an anti-CD20 antibody, which is a marker of mature B cells. Its immunosuppressive effect is mediated by the depletion of the B-cell lineage. Rituximab is a humanized antibody and can remain in circulation for months after therapy. Therefore, if present in the patient’s serum, it can bind B cells in an FC crossmatch reaction and give false-positive results.
C. Prednisone.
Rationale: Prednisone is not an antibody and does not interfere with antibodies binding to cells. Therefore, it does affect FC crossmatch results.
D. Mycophenolate mofetil.
Rationale: Mycophenolate mofetil (MMF) is an inhibitor of inosine monophosphate dehydrogenase (IMPDH), which is needed for guanine synthesis during T-cell and B-cell proliferative reactions. It is not an antibody and does not interfere with antibody binding to cells. Therefore, it does affect FC crossmatch results.
B. HLA-B and HLA-DR.
C. HLA-DR and HLA-DQ.
D. HLA-A and HLA-DR.
Rationale: The recombination frequency between two genes is directly proportional to the genomic distance between them. HLA-A and -DP are the farthest apart in the HLA region, exhibiting the highest recombination frequency. The HLA-DR and HLA-DQ genes display the tightest linkage.
Rationale: Although some drugs are nephrotoxic, they do not elicit specific T-cell responses. Recipients of organs from HLA-identical siblings are likely to require less immunosuppression.
B. Crossover event at the HLA-DQ locus.
Rationale: This is conceivable but very unlikely. Crossover events are rare, especially between HLA-DR and HLA-DQ loci, which are very tightly linked.
C. Age of the donated organ.
Rationale: Age disparity, which can affect the quality of a donated organ, should not be a significant factor in sibling donation. Age of the allograft itself is not a factor in eliciting T-cell responses.
D. The direct recognition pathway.
Rationale: The siblings are HLA identical; there is no direct recognition of nonself HLA in this case.
E. The indirect recognition pathway.
Rationale: Although the recipient and donor are 8/8 HLA matched, they are not genetically identical. Polymorphic gene products, previously unencountered by recipient T cells, can be processed by recipient APCs and presented to autologous T cells, thus eliciting an immune reaction.
Rationale: The lung does exhibit some immune privilege and is to tolerate various inhaled harmless airborne antigens; however, this does not extend to tolerance of allografts.
B. It is much worse than other solid organs due to the inflammation elicited by airborne antigens.
Rationale: Lung allografts fare significantly worse in the long term than other solid organ transplants partly due to exposure to exogenous airborne antigens and pathogens, which can initiate inflammation and allograft rejection in a previously tolerant patient (Table 15-8).
C. It is about in line with other solid organs because rejection is histocompatibility driven, and other organ-specific factors play only minor roles.
Rationale: Although HLAs and other histocompatibility factors may provide the basis of rejection, other factors can initiate rejection and have a serious impact on long-term survival.
D. All lung allografts are lost within 5 years due to bronchiolitis obliterans syndrome (BOS).
Rationale: BOS has a significant impact on lung allograft survival, although not all lung allografts are lost within 5 years due to BOS.
Immunology, Immunogenetics
Figure 15-1 Monitoring of alloantibodies by solid phase assay in a sensitized allograft recipient. The evolution of three alloantibodies through desensitization protocol, transplantation, and an infection event. DSA, Donor specific antibodies; MFI, mean fluorescence intensity; PPH, plasmapheresis; TX, transplant.
Figure 15-2 Schematic representation of the HLA locus genomic organization. This figure demonstrates the organization of the different HLA class I and class II genes on chromosome 6p21.31.