Endogenous commensal bacteria provide an additional constitutive defence against infection (p. 136). They compete with pathogenic microorganisms for space and nutrients, and produce fatty acids and bactericidins that inhibit the growth of many pathogens. In addition, commensal bacteria help to shape the immune response by inducing specific regulatory T cells within the intestine (p. 78). Phagocytes express a wide range of surface receptors that allow them to identify microorganisms. These pattern recognition receptors include the Toll-like receptors, NOD (nucleotide-oligomerisation domain protein)-like receptors and mannose receptors. They recognise generic molecular motifs not present on mammalian cells, including bacterial cell wall components, bacterial DNA and viral double-stranded RNA. While phagocytes can recognise microorganisms through pattern recognition receptors alone, engulfment of microorganisms is greatly enhanced by opsonisation. Opsonins include acute phase proteins such as C-reactive protein (CRP), antibodies and complement. They bind both to the pathogen and to phagocyte receptors, acting as a bridge between the two to facilitate phagocytosis (Fig. 4.1). Neutrophils, also known as polymorphonuclear leucocytes, are derived from the bone marrow (Fig. 4.2). They are short-lived cells with a half-life of 6 hours in the blood stream, and are produced at the rate of approximately 1011 cells daily. Their functions are to kill microorganisms directly, facilitate the rapid transit of cells through tissues, and non-specifically amplify the immune response. This is mediated by enzymes contained in granules which also provide an intracellular milieu for the killing and degradation of microorganisms. Monocytes are the precursors of tissue macrophages. They are produced in the bone marrow and constitute about 5% of leucocytes in the circulation. From the blood stream, they migrate to peripheral tissues, where they differentiate into tissue macrophages and reside for long periods. Specialised populations of tissue macrophages include Kupffer cells in the liver, alveolar macrophages in the lung, mesangial cells in the kidney, and microglial cells in the brain. Macrophages, like neutrophils, are capable of phagocytosis and killing of microorganisms but also play an important role in the amplification and regulation of the inflammatory response (Box 4.1). They are particularly important in tissue surveillance, monitoring their immediate surroundings for signs of tissue damage or invading organisms. Cytokines are small soluble proteins that act as multipurpose chemical messengers. Examples are listed in Box 4.2. They are produced by cells involved in immune responses and by stromal tissue. More than 100 cytokines have been described, with overlapping, complex roles in intercellular communication. Their clinical importance is demonstrated by the efficacy of ‘biological’ therapies (often abbreviated to ‘biologics’) that target specific cytokines (pp. 1102 and 18). The complement system is a group of more than 20 tightly regulated, functionally linked proteins that act to promote inflammation and eliminate invading pathogens. Complement proteins are produced in the liver and are present in the circulation as inactive molecules. When triggered, they enzymatically activate other proteins in a rapidly amplified biological cascade analogous to the coagulation cascade (p. 995). There are three mechanisms by which the complement cascade may be triggered (Fig. 4.3): • The alternative pathway is triggered directly by binding of C3 to bacterial cell wall components, such as lipopolysaccharide of Gram-negative bacteria and teichoic acid of Gram-positive bacteria. • The classical pathway is initiated when two or more IgM or IgG antibody molecules bind to antigen, forming immune complexes. The associated conformational change exposes binding sites on the antibodies for C1. C1 is a multiheaded molecule which can bind up to six antibody molecules. Once two or more ‘heads’ of a C1 molecule are bound to antibody, the classical cascade is triggered. • The lectin pathway is activated by the direct binding of mannose-binding lectin to microbial cell surface carbohydrates. This mimics the binding of C1 to immune complexes and directly stimulates the classical pathway. Activation of complement by any of these pathways results in activation of C3. This, in turn, activates the final common pathway, in which the complement proteins C5–C9 assemble to form the membrane attack complex. This can puncture target cell walls, leading to osmotic cell lysis. This step is particularly important in the defence against encapsulated bacteria, such as Neisseria spp. and Haemophilus influenzae. Complement fragments generated by activation of the cascade can also act as opsonins, rendering microorganisms more susceptible to phagocytosis by macrophages and neutrophils (see Fig. 4.1). In addition, they are chemotactic agents, promoting leucocyte trafficking to sites of inflammation. Some fragments act as anaphylotoxins, binding to complement receptors on mast cells and triggering release of histamine, which increases vascular permeability. The products of complement activation also help to target immune complexes to antigen-presenting cells, providing a link between the innate and the acquired immune systems. Finally, activated complement products dissolve the immune complexes that triggered the cascade, minimising bystander damage to surrounding tissues. Mast cells and basophils are bone marrow-derived cells which play a central role in allergic disorders. Mast cells reside predominantly in tissues exposed to the external environment, such as the skin and gut, while basophils are located in the circulation and are recruited into tissues in response to inflammation. Both contain large cytoplasmic granules which contain preformed vasoactive substances such as histamine (see Fig. 4.9, p. 89). Mast cells and basophils express IgE receptors on their cell surface (see Fig. 4.5). On encounter with specific antigen, the cell is triggered to release preformed mediators and synthesise additional mediators, including leukotrienes, prostaglandins and cytokines. These trigger an inflammatory cascade which increases local blood flow and vascular permeability, stimulates smooth muscle contraction, and increases secretion at mucosal surfaces. If the innate immune system fails to provide effective protection against an invading pathogen, the adaptive immune system (Fig. 4.4) is mobilised. This has three key characteristics: • It has exquisite specificity and is able to discriminate between very small differences in molecular structure. • It is highly adaptive and can respond to an unlimited number of molecules. • It possesses immunological memory, such that subsequent encounters with a particular antigen produce a more effective immune response than the first encounter. • Primary lymphoid organs. The primary lymphoid organs are involved in lymphocyte development. They include the bone marrow, where both T and B lymphocytes are derived from haematopoietic stem cells (p. 993) and where B lymphocytes also mature, and the thymus, where T lymphocytes mature. • Secondary lymphoid organs. After maturation, lymphocytes migrate to the secondary lymphoid organs. These include the spleen, lymph nodes and mucosa-associated lymphoid tissue. These organs trap and concentrate foreign substances, and are the major sites of interaction between naïve lymphocytes and microorganisms. The thymus is a bilobed structure organised into cortical and medullary areas. The cortex is densely populated with immature T cells, which migrate to the medulla to undergo selection and maturation. The thymus is most active in the fetal and neonatal period, and involutes after puberty. Failure of thymic development is associated with profound T-cell immune deficiency (p. 80), but surgical removal of the thymus in childhood (usually in the context of major cardiac surgery) is not associated with significant immune dysfunction. The spleen is the largest of the secondary lymphoid organs. It is highly effective at filtering blood and is an important site of phagocytosis of senescent erythrocytes, bacteria, immune complexes and other debris. It is also a major site of antibody synthesis. It is particularly important for defence against encapsulated bacteria, and asplenic individuals are at risk of overwhelming Streptococcus pneumoniae and H. influenzae infection (see Box 24.40, p. 1028). Lymphoid tissues are physically connected by a network of lymphatics, which has three major functions: it provides access to lymph nodes, returns interstitial fluid to the venous system, and transports fat from the small intestine to the blood stream (see Fig. 16.14, p. 452). The lymphatics begin as blind-ending capillaries, which come together to form lymphatic ducts. These enter and then leave regional lymph nodes as afferent and efferent ducts respectively. They eventually coalesce and drain into the thoracic duct and thence into the left subclavian vein. Lymphatics may be either deep or superficial, and, in general, follow the distribution of major blood vessels. Immunoglobulins (Ig) are soluble proteins made up of two heavy and two light chains (Fig. 4.5). The heavy chain determines the antibody class or isotype, i.e. IgG, IgA, IgM, IgE or IgD. Subclasses of IgG and IgA also occur. The antigen is recognised by the antigen-binding regions (Fab) of both heavy and light chains, while the consequences of antibody-binding are determined by the constant region of the heavy chain (Fc) (Box 4.3). Antibodies can initiate a number of different actions. They facilitate phagocytosis by acting as opsonins (see Fig. 4.1), and can also facilitate cell killing by cytotoxic cells (ADCC, p. 75). Binding of antibodies to antigen can trigger activation of the classical complement pathway (see Fig. 4.3). In addition, antibodies may act directly to neutralise the biological activity of toxins. This is a particularly important feature of IgA antibodies, which act predominantly at mucosal surfaces. T lymphocytes (also known as T cells) mediate cellular immunity and are important for defence against viruses, fungi and intracellular bacteria. They also play an important immunoregulatory role, orchestrating and regulating the responses of other components of the immune system. T-lymphocyte precursors arise in bone marrow and are exported to the thymus while still immature (see Fig. 4.6 below). Within the thymus, each cell expresses a T-cell receptor with a unique specificity. These cells undergo a process of stringent selection to ensure that autoreactive T cells are deleted. Mature T lymphocytes leave the thymus and expand to populate other organs of the immune system. It has been estimated that an individual possesses 107–109 T-cell clones, each with a unique T-cell receptor, ensuring at least partial coverage for any antigen encountered. CD4+ lymphocytes can be further subdivided into subsets on the basis of the cytokines they produce: • Typically, Th1 cells produce IL-2, IFN-γ and TNF-α, and support the development of delayed type hypersensitivity responses (p. 87). • Th2 cells typically secrete IL-4, IL-5 and IL-10, and promote allergic responses (p. 89). • A further subset of specialised CD4+ lymphocytes known as regulatory cells are important in immune regulation of other cells and the prevention of autoimmune disease. The consequences of deficiencies of the immune system include recurrent infections, autoimmunity and susceptibility to malignancy. Immune deficiency may arise through intrinsic defects in immune function, but is much more commonly due to secondary causes, including infection, drug therapy, malignancy and ageing. This chapter gives an overview of the rare primary immune deficiencies. More than a hundred genetically determined deficiencies have been described, most of which present in childhood or adolescence. The clinical manifestations are dictated by the component of the immune system involved (Box 4.4), but there is considerable overlap and redundancy in the immune network so some diseases do not fall easily into this classification. Most patients with an immune deficiency present with recurrent infections. While there is no accepted definition of ‘too many’ infections, features that may indicate immune deficiency are shown in Box 4.5. Frequent, severe infections or infections caused by unusual organisms or at unusual sites are the most useful indicator. Primary phagocyte deficiencies (see Fig. 4.2, p. 73) usually present with recurrent bacterial and fungal infections which may affect unusual sites. Aggressive management of existing infections, including intravenous antibiotics and surgical drainage of abscesses, and long-term prophylaxis with antibacterial and antifungal agents, is required. Specific treatment depends upon the nature of the defect; haematopoietic stem cell transplantation may be considered (p. 1017). Genetic deficiencies of almost all the complement pathway proteins (see Fig. 4.3, p. 75) have been described. Many present with recurrent infection with encapsulated bacteria, particularly Neisseria species, reflecting the importance of the membrane attack complex in defence against these bacteria. In addition, genetic deficiencies of the classical complement pathway (C1, C2 and C4) are associated with a high prevalence of autoimmune disease, particularly systemic lupus erythematosus (SLE, p. 1109). In contrast to other complement deficiencies, mannose-binding lectin deficiency is very common (5% of the northern European population). Complete deficiency may predispose to bacterial infections in the presence of an additional cause of immune compromise, such as premature birth or chemotherapy, but is otherwise well tolerated. Deficiency of the complement regulatory protein Cl inhibitor is not associated with recurrent infections but causes recurrent angioedema (p. 93). These are characterised by recurrent viral, protozoal and fungal infections (see Box 4.4). In addition, many T-cell deficiencies are associated with defective antibody production because of the importance of T cells in regulating B cells. These disorders generally present in childhood and are illustrated in Figure 4.6. The principal tests for T-lymphocyte deficiencies are a total blood lymphocyte count and quantitation of lymphocyte subpopulations by flow cytometry. Serum immunoglobulins should also be measured. Second-line, functional tests of T-cell activation and proliferation may be indicated. Patients in whom T-lymphocyte deficiencies are suspected should be tested for human immunodeficiency (HIV) infection (p. 392). Anti-Pneumocystis and antifungal prophylaxis, and aggressive management of infections, are required. Immunoglobulin replacement may be indicated if antibody production is impaired. Haematopoietic stem cell transplantation (HSCT, p. 1017) may be appropriate. Severe combined immune deficiency (SCID) is caused by defects in lymphoid precursors and results in combined failure of B- and T-cell maturation. The absence of an effective adaptive immune response causes recurrent bacterial, fungal and viral infections soon after birth. HSCT (p. 1017) is the only current treatment, although gene therapy is under investigation.
Immunological factors in disease
Functional anatomy and physiology of the immune system
The innate immune system
Constitutive barriers to infection
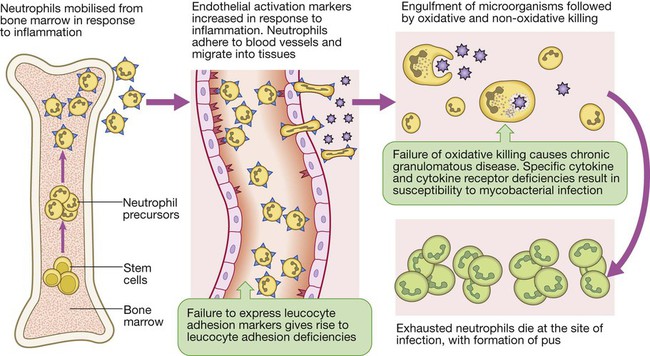
Phagocytes
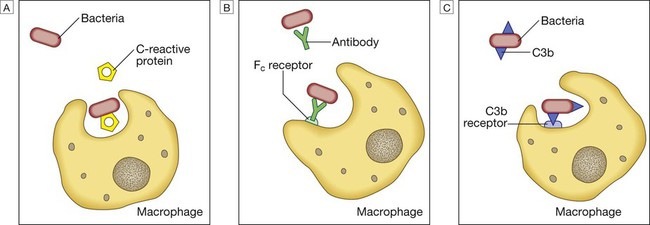
Phagocytosis of microbial products may be augmented by several opsonins. A C-reactive protein. B Antibody. C Complement fragments.
Neutrophils
Monocytes and macrophages
Cytokines
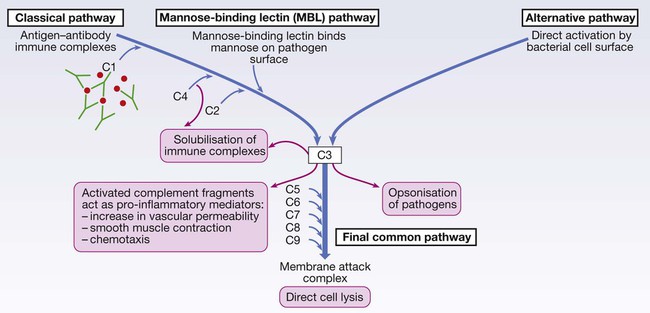
Complement
Mast cells and basophils
The adaptive immune system
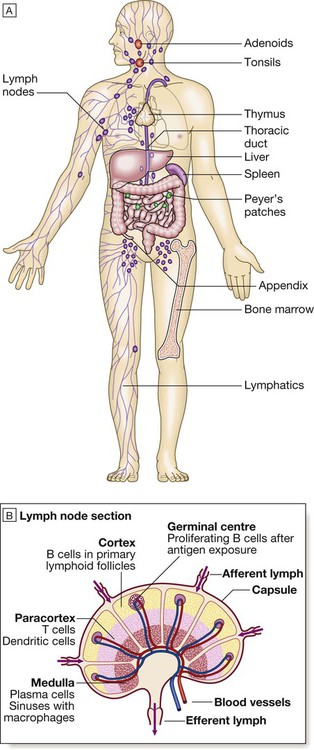
Lymphoid organs
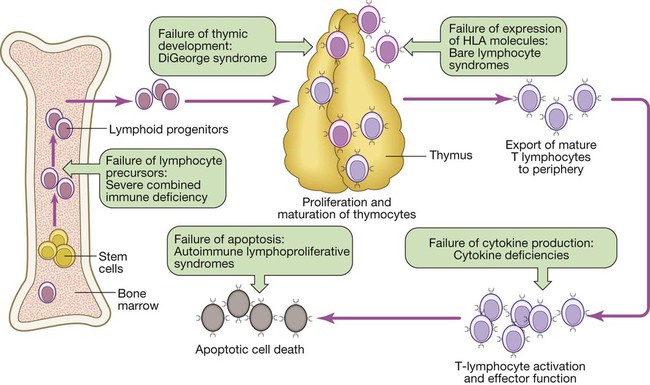
The thymus
The spleen
Lymphatics
Humoral immunity
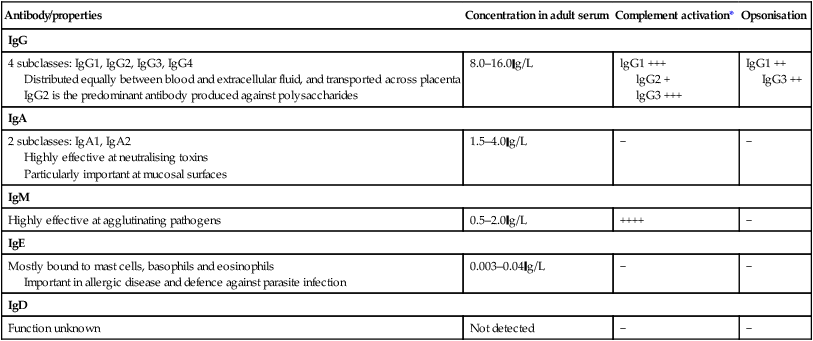
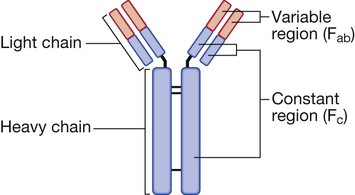
Immunoglobulins
Cellular immunity
Immune deficiency
Presenting problems in immune deficiency
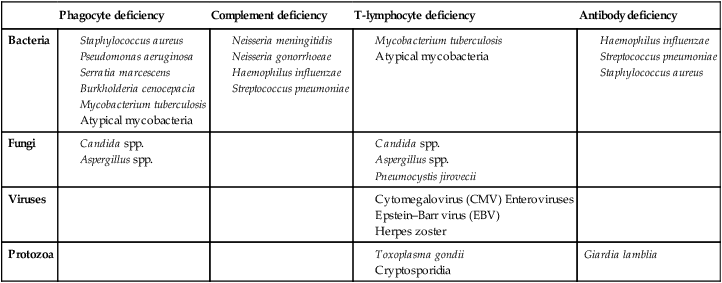
Recurrent infections
Primary phagocyte deficiencies
Complement pathway deficiencies
Primary deficiencies of the adaptive immune system
Primary T-lymphocyte deficiencies
Investigations and management
Combined B- and T-lymphocyte immune deficiencies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunological factors in disease