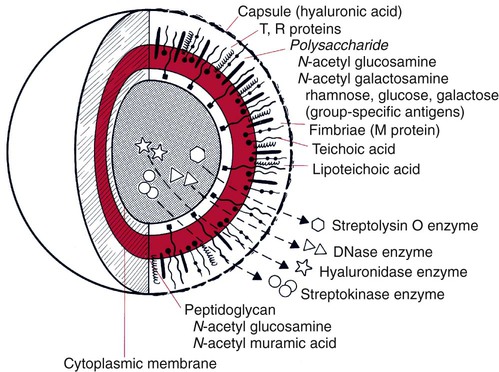
1. List the reasons a laboratory or clinician would use immunochemical tests to diagnose disease. 2. Define a polyclonal antibody and a monoclonal antibody and explain the difference between the two. 3. Explain how monoclonal antibodies are produced. How has their development affected immunochemical testing? 4. Define the four types of immunochemical testing—precipitation, particle agglutination, immunofluorescent assays, and enzyme immunoassays—and provide a clinical application for each. 5. Explain the difference between a direct fluorescent antibody (DFA) test and an indirect fluorescent antibody (IFA) test and explain how each is used in the clinical laboratory. 6. Explain the function of the hypoxanthine, aminopterin, and thymidine (HAT) medium in hybridoma production. The diagnosis of an infectious disease by culture and biochemical techniques can be hindered by several factors. These factors include the inability to cultivate an organism on artificial media, such as Treponema pallidum, the agent that causes syphilis, or the fragility of an organism and its subsequent failure to survive transport to the laboratory, such as with respiratory syncytial virus and varicella-zoster virus. Another factor, the fastidious nature of some organisms (e.g., Leptospira or Bartonella spp.) can result in long incubation periods before growth is evident. In addition, administration of antimicrobial therapy before specimen collection, such as with a patient who has received partial treatment, can impede diagnosis. In these cases, detecting a specific product of the infectious agent in clinical specimens is very important, because this product would not be present in the specimen in the absence of the agent. This chapter discusses the direct detection of microorganisms in patient specimens using immunochemical methods and the identification of microorganisms by these methods once they have been isolated on laboratory media. Chapter 10 discusses the diagnosis of infectious diseases using serological methods. Immunochemical methods use antigens and antibodies as tools to detect microorganisms. Antigens are substances recognized as “foreign” in the human body. Antigens are usually high-molecular-weight proteins or carbohydrates that elicit the production of other proteins, called antibodies, in a human or animal host (see Chapter 3). Antibodies attach to the antigens and aid the host in removing the infectious agent (see Chapters 3 and 10). Antigens may be part of the physical structure of the pathogen, such as the bacterial cell wall, or they may be a chemical produced and released by the pathogen, such as an enzyme or a toxin. Each antigen contains a region that is recognized by the immune system. These regions are referred to as antigenic determinants or epitopes. Figure 9-1 shows the multiple molecules within group A Streptococcus (Streptococcus pyogenes) that are recognized by the immune system as antigenic. Monoclonal antibodies are antibodies that are completely characterized and highly specific. The ability to create an immortal cell line that produces large quantities of a monoclonal antibody has revolutionized immunologic testing. Monoclonal antibodies are produced by the fusion of a malignant single antibody-producing myeloma cell with an antibody-producing plasma B cell, forming a hybridoma cell. Clones of the hybridoma cells continuously produce specific monoclonal antibodies. One technique for the production of a clone of cells is illustrated in Figure 9-2. In the double immunodiffusion method, small circular wells are cut in an agarose gel, a gelatin-like matrix derived from agar, which is a chemical purified from the cell walls of brown algae. The agarose forms a porous material through which molecules can readily diffuse. The patient specimen containing antigen is placed in a well, and antibody directed against the antigen is placed in the adjacent well. Over 18 to 24 hours, the antigen and antibody diffuse toward each other, producing a visible precipitin band (a lattice structure or visible band) at the point in the gel where the antigen and antibody are in equal proportion (zone of equivalence). If the concentration of antibody is significantly higher than that of the antigen, no lattice forms and no precipitation reaction occurs; this is known as prozone effect. Conversely, if excess antigen prevents lattice formation, resulting in no band formation, the effect is termed postzone. Immunodiffusion is currently used to detect exoantigens produced by the systemic fungi to confirm their presence in culture (Figure 9-3). However, the technique is extremely time-consuming and is no longer used regularly in the clinical laboratory for antigen detection in patient specimens.
Immunochemical Methods Used for Organism Detection
Production of Antibodies for Use in Laboratory Testing
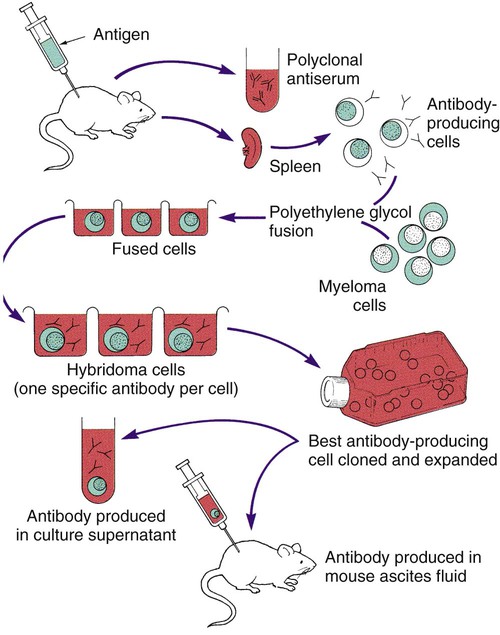
Monoclonal Antibodies
Principles of Immunochemical Methods Used for Organism Detection
Precipitation Tests
Double Immunodiffusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunochemical Methods Used for Organism Detection