Figure 49-1. Schematic illustration of different forms of simple mechanical obstruction. Simple obstruction is most often due to adhesion (A), groin hernia (B), or neoplasm (C). The hernia can act as a tourniquet, causing a closed-loop obstruction and strangulation.
1 The various forms of mechanical intestinal obstruction can be classified according to different but overlapping schemes. Most commonly, obstruction is classified according to etiology. As detailed in Table 49-1, distinctions are drawn between intraluminal obturators such as foreign bodies or gallstones, intramural lesions such as tumors or intussusceptions, and extrinsic or extramural lesions such as adhesions. Adhesions are the most common cause of intestinal obstruction, accounting for more than half of all cases. In order to highlight the pathophysiology, presentation, and natural history, however, it is useful to classify obstruction according to the location of the obstructing lesion. Proximal or “high” obstructions involve the pylorus, duodenum, and proximal jejunum. Intermediate levels of obstruction involve the intestine from the midjejunum to the midileum. Distal levels of obstruction arise in the distal ileum, ileocecal valve, and proximal colon whereas the most distant or “low” obstructions would arise in regions beyond the transverse colon. As shown in Table 49-2, clinical symptoms and signs of obstruction (pain, vomiting, abdominal distention, and gas pattern on abdominal radiographs) vary with the level of obstruction.
It is also important to distinguish between open-loop and closed-loop obstructions. An open-loop obstruction occurs when intestinal flow is blocked but proximal decompression is possible through vomiting. A closed-loop obstruction occurs when inflow to the loop of bowel and outflow from the loop are both blocked. This obstruction permits gas and secretions to accumulate in the loop without a means of decompression, proximally or distally. Examples of closed-loop obstructions are torsion of a loop of small intestine around an adhesive band (Fig. 49-2), incarceration of the bowel in a hernia, volvulus of the cecum or colon, or development of an obstructing carcinoma of the colon with a competent ileocecal valve. The primary symptoms of a closed-loop obstruction of the small intestine are sudden, severe midabdominal pain and vomiting whereas symptoms of the large intestine are pain and sudden abdominal distention. This pain often occurs before associated findings of localized abdominal tenderness or involuntary guarding. When signs of peritoneal irritation or frank peritonitis develop, there is a high level of suspicion that the viability of the bowel is compromised.
Table 49-1 Classification of Adult Mechanical Intestinal Obstructions

Pathophysiology of Intestinal Obstruction
Local Effects of Bowel Obstruction
When a loop of bowel becomes obstructed, intestinal gas and fluid accumulate. Stasis of luminal content favors bacterial overgrowth, alters intestinal fluid transport properties and motility, and causes variations in intestinal perfusion and lymph flow. Luminal contents and volume, bacterial proliferation, and alterations in motility and perfusion work in concert to determine the rate at which symptoms and complications develop. Each of these factors merits discussion in some detail.
Intestinal Gas. Approximately 80% of the gas seen on plain abdominal radiographs is attributable to swallowed air.6 Approximately 70% of the gas in the obstructed gut is inert nitrogen.10 Oxygen accounts for 10% to 12%, CO2 for 6% to 9%, hydrogen 1%, methane 1%, and hydrogen disulfide 1% to 10%. In the setting of acute pain and anxiety, patients with intestinal obstruction may swallow excessive amounts of air. Passage of such swallowed air distally is prevented by nasogastric suction.
Intestinal Flora. An important contribution to normal digestive function comes from its bacterial population.11 In patients with normal gastric acid secretion, the chyme entering the duodenum is sterile. The small numbers of bacteria that are found in stomach and proximal intestine are aerobic, gram-positive species found in the oropharynx. Distally, in the ileum and colon, gram-negative aerobes are present and anaerobic organisms predominate. Total bacterial counts in normal feces reach 1011 organisms per gram of fecal matter. Control of the bacterial populations depends on intact motor activity of the intestines and the interactions of all species present. This ecology can be disturbed by antibiotic therapy or by surgical reconstructions that result in stasis within intestinal segments. Intestinal bacteria serve several functions, including metabolism of fecal sterols, releasing the small-chain fatty acids that are an important food source for colonocytes; metabolism of fecal bile acids, fat-soluble vitamins (e.g., vitamin K) and vitamin B12; and breakdown of complex carbohydrates and organic matter, leading to formation of CO2, H2, and CH4 gases.9 Considerable evidence suggests that the normal flora may contribute to baseline levels of intestinal secretion and, perhaps, normal intestinal motility. Under baseline conditions, the small intestines in germfree animals are frequently dilated, fluid filled, and without peristalsis.12,13
Table 49-2 Symptoms and Signs of Bowel Obstruction


Figure 49-2. Schematic illustration of a closed-loop obstruction. The small intestine twists around its mesentery, compromising inflow and outflow of luminal contents from the loop. Also, the vascular supply to the loop may be compromised due to the twisting of the mesentery. The risk of strangulation is high.
In recent years, the role of bacterial toxins in mediating the mucosal response to obstruction has received increasing attention. In germfree dogs, luminal accumulation of fluid is not observed and absorption continues.13 In addition, it is well recognized that bacterial endotoxins can stimulate secretion, possibly via release or potentiation of activity of neuroendocrine substances and prostaglandins.12 Finally, since a substantial part of systemic microvascular and hemodynamic responses to endotoxemia appear to be attributable to heightened synthesis of nitric oxide,14,15 it seems likely that mucosal response to local inflammation and endotoxin release will also be altered by conditions modifying the synthesis or activity of nitric oxide. The role of nitric oxide in mucosal fluid and electrolyte movements is currently under active investigation.16,17
Intestinal Fluid. Classical experimental studies established that fluid accumulates intraluminally with open- or closed-loop small intestinal obstruction.9,11,18 Factors contributing to the accumulation of fluid include intraluminal distention and pressure, release of prosecretory and antiabsorptive hormones and paracrine substances, changes in mesenteric circulation, and elaboration and luminal release of bacterial toxins. Experimental studies and clinical investigation19,20 demonstrated that elevation of luminal pressures above 20 cm H2O inhibits absorption and stimulates secretion of salt and water into the lumen proximal to an obstruction. In closed-loop obstructions, luminal pressures may exceed 50 cm H2O and may account for a substantial proportion of luminal fluid accumulation.21 In simple, open-loop obstructions, distention of the lumen by gas rarely leads to luminal pressures higher than 8 to 12 cm H2O.22 In open-loop obstructions, the contributions of high luminal pressures to hypersecretion may not be important.
The release of endocrine/paracrine substances remains relatively uncharacterized in states of mechanical bowel obstruction.23,24 Suggestions have been made that vasoactive intestinal polypeptide (VIP) may be released from the submucosal and myenteric plexuses within the gut wall, promoting epithelial secretion and inhibiting absorption.24,25 Use of prostaglandin synthesis inhibitors has also implicated excess release of prostaglandins.23 Further work may be expected to focus on the role of luminal factors such as irritative bile acids, proinflammatory agents such as endotoxin and platelet-activating factor,26–28 and messengers such as nitric oxide29,30 in coordinating responses of mucosal secretory and absorptive functions during intestinal obstruction.
Intestinal Blood Flow. Microvascular responses to intestinal obstruction may also play an important role in determining the hydrostatic gradients for fluid transfer across the mucosa into the lumen. In response to heightened luminal pressure, total blood flow to the bowel wall may initially increase.31 The breakdown of epithelial barrier structures and enzymatic breakdown of stagnant intestinal contents leads to increased osmolarity of luminal contents. In addition to secretory stimulation and absorptive inhibition of the mucosa, the simultaneous changes in hydrostatic and osmotic pressures on the blood and lumen sides of the mucosa favor flow of extracellular fluid into the lumen. Perfusion is then compromised as luminal pressures increase, bacteria invade, and inflammation leads to edema within the bowel wall.11
Intestinal Motility. Obstruction of the intestinal lumen does not simply block distal passage of luminal contents. The accumulation of fluid and gas in the obstructed lumen also elicit changes in myoelectrical function of the gut, proximal and distal to the obstructed segment. In response to this distention, the obstructed segment itself may dilate, a process known as receptive relaxation.32 Such changes ensure that, despite accumulation of air and fluid, intraluminal pressures do not amplify easily to the point of compromising blood flow to the intestinal mucosa. At sites proximal and distal to the obstruction, changes in myoelectrical activity are time dependent. Initially, there may be intense periods of activity and peristalsis. Subsequently, myoelectrical activity is diminished and the interdigestive migrating myoelectrical complex pattern, is replaced by ineffectual and seemingly disorganized clusters of contractions.33–35 Similar alterations have been observed in experimental models of large bowel obstruction. Subsequent patterns of myoelectrical quiescence may correspond to increasing accumulation of fluid and air proximally and the attempt to prevent luminal pressures from rising. It is likely that many factors contribute to the rate at which these changes in myoelectrical activity occur.36 These factors would include neurohumoral milieu, bacterial products, and luminal constituents.
Complications and Systemic Effects of Bowel Obstruction
Closed-Loop Obstructions. The complications of closed-loop obstructions evolve rapidly. The reasons for this rapid evolution are best understood by considering the simplest and most common form of closed-loop obstruction, appendicitis. When a fecalith obstructs the blind-ended appendix, secretion of mucus and enhanced peristalsis represent the initial attempt to clear the blockage. Intense crampy abdominal pain focused at the umbilicus results. Nausea and vomiting are not uncommon as a result of luminal obstruction but as a reflexive response to hyperperistalsis and stretching of the mesentery. Over the next 8 to 18 hours, continued secretion of mucus to high intraluminal pressures, stasis, bacterial overgrowth, mucosal disruption, and elevation of luminal pressures convert intermittent cramps to constant and worsening pain. When luminal pressure exceeds mural venous pressure and then capillary perfusion pressures, inflammatory cells are recruited from surrounding peritoneal structures. This sequence of events leads to intense inflammation, release of exudate in the area of the appendix and the first localization of pain from the umbilicus to the area of peritoneum lying nearest the inflamed appendix. Peritoneal findings (localized tenderness, involuntary guarding, rebound, or referred tenderness) and fevers appear. Subsequently, 20 to 24 hours into the illness, the blood supply of the appendix is compromised. Gangrene and perforation follow and, if not contained by surrounding structures, free perforation leads to a rigid abdomen. Toxins from necrotic tissue and bacterial overgrowth are released into the systemic circulation and shock ensures. Torsion of a loop of small intestine around an adhesive band or inside a hernia leads to a similar pattern of events. As discussed below, torsions of the large bowel are usually accompanied by massive distention of the loop by air and feces, but the compromise of intestinal wall perfusion and evolution into peritonitis, systemic toxicity, and shock are similar.
Open-Loop Obstructions. Complications in open-loop obstructions do not necessarily evolve as rapidly as in closed-loop obstructions. Not uncommonly, an open-loop obstruction located in the proximal jejunum can be decompressed by the patient’s ability to vomit. Proximal obstruction is characterized by vomiting and loss of gastric, pancreatic, and biliary secretions, with resulting electrolyte disturbances. These disturbances include dehydration, metabolic alkalosis, hypochloremia, hypokalemia, and usually hyponatremia. In contrast, obstructions of the distal ileum may lead only to a slowly progressing distention of the small intestine, with accommodation by intestinal myoelectrical function and minor alterations in fluid and electrolyte balances. Open-loop obstructions located in the midgut are often complicated by events similar to those seen in closed-loop obstructions or combinations of events seen in high and low obstructions (Table 49-2). Patients with distal jejunal obstruction tend to present with a combination of complications resulting from loss of intestinal contents from vomiting, as well as distention and compromise of intestinal wall perfusion.
2 In simple or uncomplicated obstruction, the intestinal lumen is partially or completely occluded without compromise of intestinal blood flow. Simple obstructions may be complete, meaning that the lumen is totally occluded, or incomplete, meaning that the lumen is narrowed but permitting distal passage of some fluid and air. In strangulation obstruction, blood flow to the obstructed segment is compromised and tissue necrosis and gangrene are imminent.
Clinical Presentation and Differential Diagnosis
3 The four key symptoms associated with acute mechanical bowel obstruction include abdominal pain, vomiting, distention, and obstipation. When bowel obstruction is the most likely diagnosis, “abdominal pain out of proportion to physical findings” represents a surgical emergency. Colon obstruction is usually accompanied by varying levels of pain with massive abdominal distention and obstipation. As noted earlier, the signs and symptoms of acute but simple small intestinal obstructions are related to the level of the obstruction and the closed- or open-loop nature of the obstruction. Other abdominal conditions, such as appendicitis, diverticulitis, perforated peptic ulcer, cholecystitis, or choledocholithiasis can usually be distinguished from SBO, by clinical examination and basic laboratory data. It should be emphasized that bowel obstruction can complicate any of these abdominal conditions. The presence of another abdominal process does not exclude the complication of SBO.
Over the years, numerous attempts have been made to use groupings of clinical criteria to establish the diagnosis of complete and irreversible intestinal obstruction, as distinguished from partial intestinal obstruction that might improve without operative intervention or other abdominal pathology. In recent studies, computer-assisted analysis has been used to identify such criteria.37 Key factors in the history and clinical examination38 include previous abdominal surgery, quality of pain (colic/intermittent versus steady), abdominal distention, and hyperactivity of bowel sounds. Not surprisingly, the use of such computer-assisted algorithms confirms that the most important clues to the diagnosis of simple obstruction of the small intestine are obtained in a complete and careful history and physical examination. As discussed below, the role of plain abdominal radiographs and other imaging studies is to confirm the clinical diagnosis of simple obstruction. It should be emphasized that, in simple obstruction, laboratory studies do not play a direct role in diagnosis, but aid in understanding the extent of complications such as dehydration, strangulation, and sepsis.
Strangulation obstruction is accompanied by symptoms and signs suggesting peritonitis, large fluid shifts, or systemic toxicity. These symptoms and signs include abdominal tenderness or involuntary guarding localized to the area of the strangulated loop of bowel, decreasing urine output, fever, and tachycardia. There have been attempts to use common clinical and laboratory test criteria to identify the likelihood that the obstruction is associated with strangulation. Stewardson et al.39 observed that the risk of strangulation was low in patients with partial (i.e., incomplete) or complete SBO if fever, tachycardia, localized abdominal tenderness, or leukocytosis were not present. These authors suggested that, in a setting consistent with bowel obstruction, any one of these four cardinal signs indicated a small risk for strangulation. The presence of any two of these signs increased the risk of strangulation so high as to warrant immediate surgery. These authorities and others have stressed, however, that when complete obstruction is present, no satisfactory clinical criteria are available to reliably exclude the possibility of strangulation.39–43
Different laboratory tests have been advocated for early detection of strangulated intestine. Metabolic (i.e., lactic) acidosis and increases in serum amylase, inorganic phosphate, hexosaminidase, intestinal fatty acid–binding protein, and serum D-lactate levels have all been associated with intestinal ischemia.44,45 Such laboratory abnormalities may be helpful in diagnosing established strangulation in a small group of patients where the diagnosis of necrotic bowel is not clear. However, a noninvasive and rapid test has not yet been developed that can provide information to suggest that tissue necrosis is imminent but not yet established.43
Radiographs and Imaging
Plain Films
The role of plain abdominal radiographs and imaging studies is to confirm the diagnosis of bowel obstruction, locate the site of obstruction, and gain insight into the lesion responsible for the obstruction. On plain radiographs of the abdomen, the key findings of SBO reflect the accumulation of air and fluid proximal, and clearance of fluid and air distal to the point of obstruction. Dilated loops of small intestine are defined as those greater than 3 cm in diameter. Free air represents perforation of a viscus and mandates immediate operation. Such findings include dilated loops of small bowel on the flat plate (Fig. 49-3) and multiple air–fluid levels located at different levels on the upright film or lateral decubitus film (Fig. 49-4). Based on these criteria plain abdominal radiography is diagnostic in 67% to 80% of patients.46,47 Plain radiographs, however are only accurate in 46% to 85% of the time and can miss SBO in patients without air–fluid levels because of fluid-filled distended loops.48 In complete obstruction of the small intestine, the colon loops and rectum do not contain air. If there is air in the colon, the obstruction may be complete, but early, or it may be incomplete.
In the colon, tight closed-loop obstructions (i.e., volvulus of the cecum, transverse colon, or sigmoid colon) are accompanied by distention of the obstructed segment (Fig. 49-5). The proximal colon is considered dilated when it reaches 8 to 10 cm; the sigmoid colon is considered dilated at 4 to 5 cm. In contrast, obstruction by carcinoma or diverticulitis presents with massive distention of the entire colon from the point of obstruction to the ileocecal valve. From this standpoint, any large bowel obstruction may represent a “closed loop” if the ileocecal valve is competent. Although plain film findings can be used to differentiate obstruction of the small bowel from that of the large bowel, they are not consistently accurate in localizing the specific site of obstruction.

Figure 49-3. Plain supine abdominal film of a patient with small-intestinal obstruction. Note the multiple dilated loops of small intestine (black arrow) in the left upper quadrant, characterized by complete markings of the plicae. Also note the absence of air in colon and rectum.
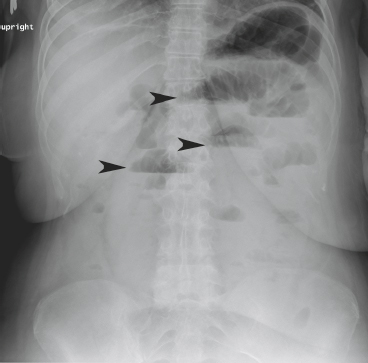
Figure 49-4. Plain upright abdominal film of a patient with small-intestinal obstruction. Note the air–fluid levels in the stomach and multiple dilated loops of small intestine (black arrows), and absence of air in the colon or rectum.

Figure 49-5. Plain upright abdominal film of a patient with sigmoid volvulus. The dilated centrally located sigmoid loop is seen (arrowheads). The proximal colon is dilated and gas filled. T, transverse colon; D, descending colon.
Contrast Studies
The diagnosis of bowel obstruction can generally be made by considering the clinical history, physical examination, laboratory, and plain radiograph findings. Contrast studies (i.e., small bowel follow-through, enteroclysis, and contrast enema) may provide specific localization of the point of obstruction and the nature of the underlying lesion. When obstruction of the small intestine is not progressing or resolving, a small bowel follow-through may be performed to confirm the presence and location of the obstruction. Also, even under acute circumstances, diagnosis and management of colonic obstructions are generally enhanced by the use of a contrast enema. Under some circumstances, however, contrast studies are unnecessary and may be contraindicated. For example, in the classic setting of abdominal pain, nausea, vomiting, and a plain film indicating multiple air–fluid levels in the small intestine and colonic collapse, the diagnosis of acute obstruction can be made clinically. Failure to improve in a short period of time will mandate operation and contrast studies are unnecessary. When strangulation or perforations are strongly suspected, contrast studies are contraindicated.
4 The choice of contrast materials includes water-insoluble suspensions of barium and water-soluble agents such as Gastrografin® or Hypaque®. Barium studies provide the clearest images, in both small bowel studies where the contrast is given from above and colon/rectum studies in which the contrast is given by enema. If barium leaks into the peritoneum, it elicits intense peritonitis. If there is any possibility of bowel perforation or gangrene, barium should not be used. Water-soluble agents such as sodium amidotrizoate/meglumine amidotrizoate oral solution (Gastrografin®) or diatrizoate sodium/diatrizoate meglumine (Hypaque®) are hyperosmotic and can elicit fluid translocation into the gut. When the obstruction of the small intestine is incomplete the use of these agents may facilitate resolution. Gastrografin is hyperosmolar (1,900 mOsm/L). Its administration permits mobilization of fluid into the bowel lumen which decreases edema of the intestinal wall and increases the pressure gradient across obstructive site. It is thought that these fluid and pressure shifts can contribute to resolution in cases of incomplete obstruction. The 2013 Bologna guidelines for diagnosis and management of adhesive SBO recommended a dosage of 50 to 150 mL of Gastrograffin administered either orally or via NGT immediately on admission or after an attempt of conservative treatment for 48 hours.49 The appearance of contrast in the colon within 4 to 24 hours after administration had a sensitivity of 96% and specificity of 98% in predicting resolution of SBO.50 A prospective randomized trial confirmed that gastrograffin significantly reduced the need for surgery by 74%.51 Some studies showed that water-soluble contrast medium reduces hospital stay but does not reduce need for surgery52,53 but recent meta-analysis showed that it is effective in reducing the need for surgery and shortening length of stay.50 Use of water-soluble contrast may thus be both diagnostic and can be therapeutic.
Computed Tomography and Other Imaging Modalities
5 The potential benefits of computed tomography (CT) scanning in diagnosis of bowel obstruction include the following.54–57 First, using radiographic contrast, the obstructing segment may be localized and characterized as complete or incomplete. Second, the nature of the obstructing lesion, especially if it is malignant, can be established. Third, additional abdominal pathology (e.g., metastases, ascites, parenchymal liver abnormalities) may be identified. Fourth, anatomic information obtained from the CT can be used in operative planning. There is also evidence that, in special circumstances, CT may improve preoperative detection of strangulation.42,56 Coronal and sagittal reconstruction improve the ability to identify transition point and IV contrast with delayed imaging should be considered to assess for venous occlusion or delayed bowel wall enhancement.58 CT findings indicating the site of obstruction and impending ischemia include beak-like narrowing, mesenteric edema, vascular engorgement, moderate to severe intestinal wall thickening (greater than 2 mm) (Fig. 49-6). In addition, high attenuation of bowel wall on unenhanced CT scans, low or reduced attenuation of bowel wall on intravenous contrast CT scans, and presence of intramural air (pneumatosis) or portal venous gas are CT findings suggestive of strangulation. Attenuation reflects increased energy absorption, scattering, beam divergence, and other causes of energy loss. Thus, areas of high attenuation appear darker than areas of low attenuation. CT scan has a 96% sensitivity and 93% specificity with a negative predictive value of 99% in diagnosing intestinal strangulation.59 Although CT scan need not be routinely performed unless history, physical examination, and plain films are not conclusive for SBO diagnosis,60 it is increasingly the “go-to” study for confirmation.

Figure 49-6. Axial (A) and coronal (B) images of a closed-loop obstruction with strangulated small bowel secondary to a volvulus from an adhesive band. Distended fluid-filled loops of small bowel (B) in a radial distribution converge toward the point of torsion (white arrows). There is edema within the mesentery (M). Shown in (C) is a coronal view of vascular engorgement and mesenteric edema in a closed-loop obstruction.
Ultrasound has a very limited use in diagnosing SBO and visualization can be obscured by the intraluminal air but it has been suggested that real-time abdominal sonography could aid in the diagnosis of strangulation obstruction. In studies conducted in two different institutions, Ogata and colleagues61,62 demonstrated that the presence of significant amounts of peritoneal fluid and of an akinetic and dilated loop of bowel were strongly associated with the presence of strangulation. In patients who had strangulation, but were thought to have simple obstruction only, these findings helped to make the preoperative diagnosis of infarction.
Magnetic resonance imaging (MRI) has the sensitivity comparable to CT scan in diagnosing obstruction but limitations include lack of availability after hours, poor definition of mass lesion, and poor visualization of colonic obstructions.63 The use of MRI should be limited to patients who have contraindications to CT or are allergic to contrast material.49
It should be emphasized that when the clinical picture suggests strangulation, unnecessary imaging studies should not delay resuscitation or expeditious movement to the operating room. Such studies will not necessarily be helpful when clinical criteria and basic abdominal radiographs have indicated the presence of a simple and complete obstruction. By itself, this diagnosis mandates urgent exploration and the information sought should be weighed against the risk of delay in going to the operating room. In fact, in most studies evaluating the impact of imaging on diagnosis and timing of intervention, clinical diagnosis is seldom incorrect – it is highly specific when multiple clinical signs (tenderness, peritoneal signs, leukocytosis, and profound dehydration) of strangulation are present. However, such a picture represents advanced disease and use of CT scan may detect strangulation before such signs are manifest. These findings reinforce the dictum that when there are clinical signs of strangulation, surgery should be performed without delaying for additional imaging studies. In patients with equivocal findings or uncertain clinical diagnosis, CT can be highly useful in confirming the diagnosis, localizing the site and detecting the cause of intestinal obstruction and strangulation.56
General Considerations in Management of the Patient with Bowel Obstruction
Patients with obstruction of the large bowel present with abdominal pain, distention, and obstipation. Vomiting and electrolyte imbalances are sometimes prominent, though usually delayed. Elderly patients, in particular, are prone to dehydration. The presentation of SBO depends on level of obstruction, open- or closed-loop nature, and interval since onset of symptoms. Symptoms and signs of pain, vomiting, obstipation, and distention are present in variable degrees. The overall picture, however, is usually one of a patient with abdominal symptoms that are evolving and getting worse. In the settings described previously, the following questions must be addressed as expeditiously as possible:
1. Is the abdominal pain disproportionate to the physical findings and laboratory studies?
2. How rapidly the symptoms and signs are evolving: minutes, hours, or less acutely?
3. Does the patient suffer from dehydration, electrolyte imbalance and acid–base disturbance?
4. Is the obstruction complete or incomplete?
5. Is there a possibility of strangulation?
Clinical data and basic laboratory studies will provide reliable information to answer the first three questions. Answering questions 4 and 5 will often depend on close clinical observation and reexamination in the first hours or days after presentation. Abdominal radiographs and imaging studies are frequently used to provide additional information to help answer these latter questions, as well as providing information to identify the obstructing lesion.
Summarized in Table 49-3 are thumbnail sketches of different, but typical, kinds of patients presenting with symptoms and signs consistent with obstruction of the small intestine. The principles of diagnosis and management of each of these patients begins with clinical information that indicates the likelihood of a bowel obstruction. Laboratory studies and plain abdominal films are used to confirm the diagnosis of obstruction and determine the extent of physiologic impairment. The patient’s history and clinical course in the first few hours of observation are used to determine the likelihood of strangulation. Indications for surgery include rapid evolution of symptoms and signs and the diagnosis that the obstruction is complete. Contrast or imaging studies are used only when symptoms are not evolving rapidly and when identification of the underlying lesion might alter the operative strategy (see specific lesions later).
Table 49-3 Diagnosis and Decisions in Bowel Obstruction in Three Hypothetical Patients

6 The initial management of all patients with suspected bowel obstruction includes designating the patient “NPO” and starting intravenous fluids comprised of isotonic Ringers or normal saline solutions. Restoration of fluid and electrolyte balance is a priority, often requiring frequent evaluation of serum electrolytes and pH. In rapidly evolving cases or patients with significant dehydration, an indwelling urinary catheter should be placed to monitor urine output. Invasive hemodynamic monitoring (e.g., a Swan–Ganz catheter) may be necessary to monitor the response to fluid resuscitation in patients with severe cardiac, pulmonary, or renal insufficiency.
Nasogastric decompression is indicated in most cases. The nasogastric tube, typically a 16- or 18-Fr sump tube, serves to prevent distal passage of swallowed air and minimizes the discomfort of refluxing intestinal content. The use of longer tubes has been advocated in certain settings, especially for patients with chronic but intermittent obstruction arising from Crohn disease, peritoneal carcinomatosis, radiation enteritis, or many previous laparotomies for obstruction. The underlying rationale is that advancement of the tip of the long tube to the obstructed loop would permit more effective decompression, perhaps resulting in relaxation of the loop and relief of the obstruction. A number of studies have failed to document benefits in the use of long tubes64,65 in helping to resolve partial intestinal obstruction or prolonged ileus. Recently. A more possibly effective version of the long tube has been described, the ileus tube (Create Medic, Tokyo), which is made of silicon is 300 cm in length, is 16 Fr wide and has three channels. This tube is advanced with endoscopic guidance and has been reported in one study to be more effective (89.6 vs. 46.7%) compared to NGT group, specifically with respect to the intervals to resolution of symptoms and radiographic findings.66 Caution should be undertaken on the potential risk of development of pneumonia and respiratory failure in routine use of NGT.67
A randomized controlled trial suggested that resolution of partial SBO could be accelerated with the use of a regimen of oral adjunctive therapy that included magnesium oxide (500 mg), simethicone (40 mg) and Lactobacillus acidophilus (0.3 g tablet) given three times daily via NGT was effective in hastening the resolution of the conservatively treated partial adhesive SBO and shortening the hospital stay with no difference in complication rate and recurrence. Magnesium oxide was used due to its laxative side effects. Simethicone, a defoaming agent, was used because it alters surface tension of gas bubbles and causes them to coalesce, thereby accelerating the passage of gas through the intestinal tract. Lactobacillus, a probiotic, may have multiple benefits, including improved digestion and prevention of overgrowth of pathogenic bacterial species.67a
Recent study showed the potential beneficial effects of hyperbaric oxygen (HBO) therapy at a pressure of 2.0 atmospheres absolute with 100% oxygen given once a day up to 7 days. HBO therapy was associated with earlier resumption of oral intake (4.7 days vs. 5.6 days; p = 0.001), shorter hospital stay (10.3 days vs. 14.1; p = 0.001) and lower operative rate (7.4% vs. 14.8%).68 It is postulated that intestinal edema is decreased through the osmotic effect of oxygen. Oxygenation under high pressure enhances inert gas diffusion from the closed intestinal lumen into the blood. Relaxation of the distended intestinal loop improves the compromised microcirculation and oxygenation of hypoxic intestinal tissue which leads to preservation of intestinal viability and recovery of motility. This modality may be an option in the management of patients with high anesthesiologic risk.69 A current study (HOT Trial) is underway to find out the clinical value of HBO therapy in obstruction due to radiation-induced fibrosis (ISRCTN 86894066).
The use of intravenous antibiotics in the initial management of bowel obstruction should be discussed as well. Clinically, it has been recognized that antibiotics can ameliorate the evolution of symptoms and signs of strangulation in closed-loop obstruction and appendicitis. Studies in humans have demonstrated that, even in simple obstruction, bacterial counts in succus rise from under 106 organisms/L to over 109 organisms/L70 and are not necessarily reduced with short-term administration of antibiotics.71 Moreover, experimental studies indicate that bacteria can translocate across the intestinal mucosa, passing into lymph channels.72 Further studies have demonstrated that germfree animals can survive strangulation obstruction longer than normal animals and that luminal fluid taken from obstructed segments in germ-free animals is much less toxic than fluid taken from normal animals.73,74
For all these reasons, it is a well-established practice to administer antibiotics perioperatively, in order to reduce wound infection and abdominal sepsis rates in patients undergoing operation to relieve intestinal obstruction, simple or strangulated. Once the decision has been made to proceed with surgery, broad-spectrum antibiotics, covering gram-negative aerobes and anaerobes should be given. A second-generation cephalosporin or a combination of a first-generation cephalosporin and metronidazole is a rational practice for perioperative coverage in both simple and strangulation obstruction. Nevertheless, the use of antibiotics in patients who have not yet been committed to operation has not been evaluated systematically. Giving antibiotics to patients who are being observed can obscure the underlying process and, in the end, delay optimal therapy.
The decision to perform abdominal exploration to relieve intestinal obstruction should be made expeditiously, but not in the absence of critical information or before adequate resuscitation (Algorithm 49-1). Indications for surgery are outlined in Table 49-3, for each of the thumbnail vignettes. It should be emphasized that once a diagnosis of complete obstruction is made, simple or strangulated, the operation should proceed without undue delay. It is reasonable to commit the patient to a period of observation when the diagnosis is uncertain (i.e., there is a possibility of a nonsurgical diagnosis or that the obstruction is not complete). A practical point is that obstruction occurring in a patient without a previous history of laparotomy is not likely to be caused by peritoneal adhesions. This is known as de novo obstruction and whatever the underlying cause will not usually resolve without operation.
Specific Types of Bowel Obstruction
Chronic Adhesions
As noted previously, peritoneal adhesions account for more than half of SBO cases. Lower abdominal procedures such as appendectomy, hysterectomy, colectomy, and abdominoperineal resection are common precursor operations to adhesive obstruction. Adhesions form after any abdominal procedure, however, including cholecystectomy, gastrectomy, and abdominal vascular procedures. In long-term follow-up, about 5% of patients undergoing laparotomy will develop adhesive obstruction; of these, 10% to 30% will suffer from additional episodes.75,76 Up to 80% episodes of SBO due to adhesions may resolve nonoperatively.39,40,53,64 However, an index episode and three recurrences indicate a likelihood of over 80% that there will be more recurrences.75 Surgical management of an acute episode appears to reduce subsequent recurrence rates from ∼15% to ∼6%,76 but no studies have been able to establish whether the immediate benefit of laparotomy for any given episode of simple adhesive obstruction outweighs the overall benefit of expectant management and operation only for serial recurrences. Thus, a previous history of a laparotomy simply provides a reasonable basis for expectant management of patients in whom it is not yet possible to diagnose a complete obstruction. Ultimately, patients who present with signs and symptoms of bowel obstruction are managed according to the CT findings and clinical course.

Algorithm 49-1. Algorithm for the management of adhesive small bowel obstruction.
The pathobiology of adhesion formation has been the subject of considerable investigation.11 Histologic examination of chronic adhesions reveals foreign body reaction, usually to talc, starch, lint, intestinal content, or suture. Talc and starch are found less commonly now than previously, because of improvements in techniques of manufacture and sterilization of surgical gloves. Mesothelial cells are the presumed origin of tissue plasminogen activator (TPA). TPA binds fibrin and plasminogen, thereby preventing adhesion formation. In early studies, inflammatory cells, including mast cells,77 were implicated in the process that produces adhesions. Recent studies78,79 have emphasized the role of various cytokines in exacerbating or inhibiting adhesion formation in different animal models. Biologically active substances that might prove useful in preventing postoperative adhesions include transforming growth factor beta (TGF-beta) and vascular endothelial growth factor (VEGF), both of which may be targeted for inhibition without less fear of compromising the response to bacterial infection.78,79
Current strategies to prevent adhesions after a first laparotomy include targeting the fibrinolytic system, which enhances rapid healing and appears to minimize formation of peritoneal adhesions.78 Attempts to minimize or prevent adhesion formation have resulted in development of hyaluronic acid–carboxymethylcellulose membrane (Seprafilm, Genzyme, Cambridge, MA). This compound mechanically prevents adhesion formation by physically separating adjoining tissues. It is absorbed by the body in 7 days and thus is present only during the phase of fibrosis, and not as a persistent foreign body. Randomized trials have suggested that this compound prevents, minimizes severity, and decreases density and vascularity of adhesions.78,80 Other trials and review of 17 RCT corroborate decreased incidence, extent, severity, density, and vascularity of adhesions but not total prevention of adhesions or reduction of the incidence of subsequent bowel obstruction,81 and caution not be placed on anastomosis due to increased rate of anastomotic leak.82 Seprafilm and similar barriers have been advocated for use in patients in whom a second abdominal procedure is planned or significant adhesions anticipated (e.g., Hartmann procedure, ileal pouch anal anastomosis with protecting ileostomy, pelvic surgery, gynecologic procedures, staged hepatic surgery and colon surgery) – though it has been proposed for all surgeries by the manufacturers. The largest randomized, single blind, controlled study on Seprafilm showed that the incidence of adhesive small bowel requiring reoperation was significantly lower for Seprafilm patient with absolute reduction rate of 1.6% and relative reduction rate of 47%.83
The Prevention of Postoperative Adhesion (POPA) study uses 2,000 icodextrin 4% solution before abdominal closure and follow-up upto 10 years showed that it is safe and reduces intra-abdominal adhesion formation and the risk of reobstruction (2.19% vs. 11.1%).84 Small trials showed the potential use of hydrogen adhesion barrier spray85 and lyophilized human peritoneal membrane.86
More important than pharmacologic approaches, however, are the efforts of the surgeon to pay meticulous attention to hemostasis and surgical technique, the avoidance of excessive tissue dissection, and careful search and removal of any extraneous material. The use of laparoscopic approaches, when feasible, should lower the likelihood of pathological adhesions as well.87
Early Postoperative Adhesions
Obstruction in the immediate period following abdominal surgery is uncommon but may occur in up to 1% of patients in the 4 weeks following laparotomy. Adhesions are responsible for approximately 90% of such cases and hernias for approximately 7%.88 Intussusception, internal herniation, inflammation, abscess, intramural intestinal hematoma or technical errors may be responsible for the remainder of cases.89–91 Most cases occur after surgery of the colon, especially abdominoperineal resections, or operations in the lower abdomen. It is rare for upper abdominal surgery to cause such obstructions. A common scenario is that a patient will undergo colectomy uneventfully, pass flatus and have bowel sounds by postoperative day 3. On the fourth postoperative day the patient suddenly becomes distended and uncomfortable, and stops passing flatus and stool. Patients with acutely evolving symptoms and signs represent complete obstruction and should be managed as such. In this latter setting, the mortality may be as high as 15% due to delays in recognition and operative intervention. The loss of bowel sounds after a short period of normal or hyperactive activity is worrisome for ischemia of the obstructed segment. The vast majority of such cases may be treated as partial intestinal obstruction; nasogastric suction and intravenous fluids will help resolve symptoms within a few days (Algorithm 49-2). When the clinical course does not demand earlier intervention, a nonoperative approach may be tried for 10 to 14 days and will resolve the obstruction in over 75% of such cases.88,90,92 It is recently noted that patients who experienced early postoperative adhesions may have a higher risk of developing adhesive SBO (26.5% vs. 7.5% at 5 years; p < 0.001).91
Bowel Obstruction after a Bariatric Procedure
The obesity epidemic sees the growth in the number of bariatric procedures which includes gastric banding, sleeve gastrectomy, gastric plication, roux-en-y gastric bypass, and biliopancreatic diversion with or without duodenal switch. Band slippage can occur in 15% to 20% of patients and typically presents with a history of upper abdominal symptoms of reflux, regurgitation, and dysphagia. Upper GI study is the preferred method of diagnosing band slippage showing the evidence of malposition of the band and proximal pouch dilatation with obstruction. The initial management is urgent band deflation using Huber needle and if this is unsuccessful or patient is acutely unwell, necrosis, abscess, or erosion must be considered which may require surgery. Intestinal obstruction occurs in 4.4% after roux-en-y gastric bypass. The etiology of obstruction includes internal hernia (53%), roux limb compression due to scarring (20%), adhesion (14%), stricture at gastrojejunal anastomosis, kinking of the alimentary limb, incisional hernia, and intestinal intussusception. The most common site of internal herniation is mesojejunal mesenteric window, followed by Petersen window and the mesocolic window.93 It is paramount to differentiate bariatric from nonbariatric patients presenting with SBO since there are significant differences in their management. Nonoperative management was successful in 72% of non-postbariatric patients but surgery was performed in about 62% of postbariatric surgery patients with SBO. Also, the bariatric group is most likely to undergo laparoscopy (5% vs. 2%), abdominal wall reconstruction (38% vs. 9%) and is less likely to require colostomy (1% vs. 13%). The bariatric group underwent surgery sooner within an average of 24 hours compared to 3.3 days in non-postbariatric patients.94

Algorithm 49-2. Approach to the management of malignant bowel obstruction.

Figure 49-7. Computed tomography images of an inguinal hernia. Axial (A) and coronal (B) CT scan images showing incarcerated right inguinal hernia with air- and fluid-filled loop of small bowel (arrowhead) in the right inguinal canal (arrow) causing small bowel obstruction with dilated loops of proximal small bowel (B).
Hernia
Hernias of all types are second only to adhesions as the most frequent causes of obstruction in Western countries. External hernias such as inguinal (Fig. 49-7) or femoral hernias may present with the symptoms of obstruction and will not be diagnosed unless sought.95 Femoral hernias are particularly prone to incarceration and bowel necrosis due to the small size of the hernia inlet.95 Other hernias such as umbilical, incisional, paracolostomy, or lumbar hernias are obvious. Still others, such as internal hernias are usually diagnosed at laparotomy for obstruction. These include obturator hernias, paraduodenal hernias (Fig. 49-8), and hernias through the foramen of Winslow or mesenteries. When hernia has been identified as the cause of the obstruction, the patient is quickly resuscitated, given antibiotics, and taken to the operating room. The hernia is then reduced and the viability of the bowel assessed. If viable, the bowel is left alone; if not, it is resected. The hernia defect is then repaired. One important consideration is the Richter hernia (Fig. 49-9).96 In this variant, only a portion of the wall of the bowel is incarcerated and thus incarceration and strangulation may not be associated with complete obstruction. These most frequently occur in association with femoral or inguinal hernias. Complete obstruction can occur if more than half of the bowel circumference is incarcerated.
For external (abdominal wall) hernias, it may be possible to perform taxis, that is, the manual reduction of an incarcerated/irreducible hernia. Reduction (taxis) of the hernia is usually successful. Occasionally taxis results in reduction of the contents of the hernia sac en mass (still obstructed), reduction of strangulated bowel resulting in generalized peritonitis, or reduction of an obstructed Richter hernia.97–101 This is one reason for using circumspection in relying on taxis as a mode of treatment for incarcerated inguinal, femoral, and incisional hernias. In general, taxis should be followed expeditiously by operative repair.
Gallstone Ileus
As a result of intense inflammation surrounding a gallstone, a fistula may develop between the biliary tree and the small or large intestine. Most fistulae develop between the gallbladder fundus and duodenum. If the stone is greater than 2.5 cm in diameter, it can lodge in the narrowest portion of the terminal ileum, which is just proximal to the ileocecal valve. This complication is rare, accounting for less than 6 in 1,000 cases of cholelithiasis and no more than 3% of cases of intestinal obstruction. Typically, the patient is elderly female and presents with intermittent symptoms over several days, as the stone tumbles distally toward the ileum. The classic findings on plain radiographs include those of intestinal obstruction, a stone lying outside the right upper quadrant, and air in the biliary tree (Fig. 49-10). Treatment includes removal of the stone and resection of the obstructed segment only if there is evidence of tissue necrosis. The risk of a recurrent gallstone ileus is about 5% to 10%.102,103 Such recurrences usually occur within 30 days of the initial episode and are usually due to stones in the small intestine that were missed at the original operation.
The difficult decisions in management of gallstone ileus focus on the fistula. The arguments in favor of disconnecting the fistula and removing the gallbladder have been the possibility of recurrence of gallstone ileus and the risk of cholangitis due to reflux of intestinal content into the biliary tree. When the latter operation is included, the mortality may be doubled as compared to simple removal of the gallstone. It is used selectively in good-risk patients. The long-term incidence of biliary tract infections has not been common enough to warrant the more aggressive approach at the initial operation. Some authors have advocated cholecystectomy at a second operation, especially if the patient is young and fit. The consensus is that cholecystectomy should not be performed at the initial operation for gallstone ileus, except in highly selected patients. A careful search of the entire intestine should be performed to exclude the possibility of additional large stones which can occur in up to 25% of patients.102–104
Figure 49-8. Paraduodenal hernia. A: Plain film of closed-loop obstruction with neck of the closed loop in the right upper abdomen. B: Computed tomography scan showing slippage of the jejunum behind the stomach, with dilatation and obstruction of the duodenum. C: Schematic diagram showing relationships of the paraduodenal fossa and transverse mesocolon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



