Fig. 25.1
General chart for process responsibility, product flow and communication
25.3 Competences
25.3.1 Functions of Pharmacists
Professionals dealing with medicines are engaged in the following processes: logistics, dispensing, production and control of medicines.
Logistics is about good distribution practice which includes procurement, receipt and correct storage at all stages within the chain and final delivery to pharmacies and the end user (patients) (see Chap. 36).
The dispensing of medicines is based on prescription checking (see Sect. 2.2), picking the right medicine, appropriately preparing it for patient use and instructing patients about their practical use.
The production of medicines is the prime focus of this chapter.
Control of the quality of medicines is focused on the chemical and (bio)-pharmaceutical properties and some are directly related to clinical effects of medicines in patients.
If medicines are produced on a larger scale, i.e. for more than one individual patient, more professionals will be involved.
Production will be separated from quality control, in a hospital pharmacy just as it is in pharmaceutical industry. In all areas pharmacists can be employed as well as other professionals, assistants, technicians and workers. In specific departments of the hospital pharmacy such as the production department, the sterile production department, the analytical lab, and the microbiological lab employees of other disciplines may also be employed.
25.3.2 Pharmacist in Patient Care and Product Care
Product care and patient care are the essential functions of the pharmacist at the end of the supply chain where the pharmacist meets the patient. Product care is associated with procuring (availability), storage and dispensing, including instruction of the patient of medicinal products as well as with preparation if necessary. Patient care may include counselling on the choice of medication regimens, a key part of basic academic pharmaceutical education. It may also be associated with properties of medicinal products such as efficacy and safety; or lack of safety and lack of efficacy. Healthcare professionals and patients are then the first to complain about the services delivered by the pharmacy.
The basic education of the pharmacist is directed to these responsibilities and to changes and developments in the pharmaceutical field. The education of the pharmacist has to be on an academic level to equip him with independent thinking and to guarantee a good discussion with e.g. physicians, other healthcare professionals (HCP’s) and commercially orientated managers. In addition it may be expected that scholarship from a scientific discipline is a reasonable guarantee to cope with progress in current scientific and technical knowledge of the pharmaceutical professional. Further, basic training has to be at an adequate level for further specialisation, e.g. training courses on a wide variety of subjects.
During the last decades new insights in the competences of health care professionals have evolved, centring on the communication with patients. Basic education may be pivotal in pharmacy, however insight and oversight of Healthcare institutions and the way pharmaceutical companies organise their processes are necessary as well.
The Canadian CanMEDS system [5] has inspired many basic and specialised medical and pharmaceutical courses throughout the world. This system is based on the roles and competences that a health care professional has to play in practice and by consequence provides information for an educational program. The diagram of Can MEDS has been adapted for pharmacists and is given in Fig. 25.2.
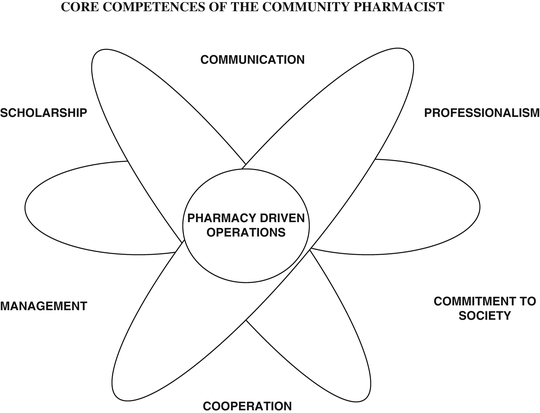

Fig. 25.2
CanMEDS diagram adapted for pharmacy (Copyright © 2005 The Royal College of Physicians and Surgeons of Canada. http://rcpsc.medical.org/canmeds. Reproduced and adapted with permission)
The different sections of Fig. 25.2 symbolise the different core competence areas of the pharmacist: pharmaceutical expertise as a pharmaceutical professional, communication with patients, cooperation with other health professionals, management as an executive, commitment to society (health advocacy) in advising patients, scholarship in an attitude towards medicines related problems.
Many academic schools use the CanMEDS framework. It is also very useful as a basis for specialised training and for continuing education. A typical example for the training of pharmacists, based on the CanMEDS-system can be found in the training of industrial pharmacists in Belgium (see Sect. 25.4.4).
Certification of health institutions such as hospitals and pharmacies for instance via ISO 9001/EN 15224 may include observations on education of HCP’s, stipulating additional programs.
25.3.3 Technicians and Assistants in Healthcare
The competences of assistants and technicians are derived from the corresponding competences of the pharmacist. In this view they perform parts of the roles of the pharmacist. If properly organised, they can be responsible for carrying out the part of the work that has been delegated to them according to the local procedures. The responsibility remains with the pharmacist. The CanMEDS diagram can be used to develop a suitable educational program for these professionals as well.
An international study about the role of pharmacy assistants and their education [6] reveals many differences and many similarities throughout Europe. About the actual preparation of medicines a variety of functions was found. It happens to be mainly a function of the assistant (e.g. in the Netherlands) or the assistant is not involved in preparation at all (in e.g. Slovenia). In some countries (e.g. in Germany) the assistants are trained to carry out chemical analysis on raw materials and pharmacy made preparations.
Technicians and assistants are working in hospital pharmacies as well. In general the same educational program can lead to competences in both community and hospital pharmacy.
25.3.4 Qualified Person
25.3.4.1 Functions
A Qualified Person (QP) has a key position in the pharmaceutical industry and it is that person who releases the batches for the market. The QP deals with quality related complaints and is leading in recall operations.
European Directive 2001/83/EC [7], as amended, states that the QP in the pharmaceutical industry has the responsibility for ensuring that a particular batch has been manufactured in accordance with its marketing authorisation, EU Good Manufacturing Practice (GMP) and equivalent regulations. Other tasks are:
Starting materials compliance and supply chain security
Manufacturing and testing performance
Manufacturing and testing process validation
Changes and investigations completion
Duties of the QP are described in more detail in Volume 4 Good manufacturing practice (GMP) Guidelines, respectively Annex 16 to GMP (see Table 25.1).
Table 25.1
Duties of the Qualified Person
Duties | Reference to GMP |
|---|---|
Release | Chapter 1.4 (xv) and 1.9 (vii) |
Producing a quality review | |
Specific place in hierarchy; relation to heads of production, quality control, quality assurance, quality unit | Chapter 1.4 (iv,v) Chapter 1.5 |
Description of duties: | Chapter 2.6 |
Ensure compliance with Marketing Authorisation (MA) | |
Permanently at disposal of the Manufacturing Authorisation holder | |
Handling unplanned deviations | Annex 16 (draft) 5 |
Contract manufacture | Annex 16 (draft) 2.3.3. |
Chain quality | Annex 16 (draft) 3.5.5 |
Handling complaints | Chapter 8.1 |
Product recalls | Chapter 8.9 |
The formal function of a QP exists in Europe and is legal for manufacturers of licensed medicines and investigational medicinal products.
PIC/S GPP ([8], see also Sect.25.5.5) acknowledges a similar function for preparation in pharmacies: Responsible Person or Releasing Officer:
6.5 Release
1. The Responsible Person is ultimately responsible for the quality of the medicinal products prepared and released. The actual release can be delegated to another appropriately competent person (i.e. Releasing Officer).
2. Product release should include verification that the medicinal products comply with the valid specifications and that they have been prepared in accordance with valid procedures and with the principles of Good Practices for preparation described in this Guide.
The title, Responsible Person, is also used in the guidelines on Good Distribution Practices of the European Commission [2]
An extensive regulation of the Qualified Person has evolved in recent years because of the important implications resulting from his duty. The role may be considered within the pharmaceutical company formally as that of a watchdog (as it is legally), by assuring that released products meet the specifications from the Marketing Authorisation.
History and Position of the QP
Until about 1900 pharmacists prepared all medicinal products in their own pharmacy,. mostly with help of self-trained assistants. With the upcoming pharmaceutical industry more complex organisations came to life and more pharmacists were needed apart from the pharmacist/owner. The introduction of managers and administrative as well as specific technical personnel led to management structures as shown in Fig. 25.1. Most of the scientific positions within the pharmaceutical industry are typically suited for pharmacists. In case of lack of interest of pharmacists to work in a pharmaceutical company a shortage of scientific personnel may have been filled up with chemists, biologists and doctors. Their specific knowledge and scientific abilities cannot be missed in the pharmaceutical industry any more. The QP needs typical pharmaceutical knowledge and abilities. If he is not a pharmacist, additional courses, based on the pharmaceutical education, are available to meet the regulations of the EC. If the QP is a pharmacist by education, he can additionally represent the company towards colleague-pharmacists, doctors and patients as a healthcare professional.
Some countries in Europe, such as the Netherlands have installed a system of administrative penalties for the company in relation to non-compliances as well as the possibility of bringing the company into court when a criminal offence is suspected. A QP could be involved as well. Jail as a penalty is common in other countries, such as Ireland.
25.3.4.2 QP and Quality Systems
The principle of Quality Management has evolved, which has assisted the work of the QP. The European approach in nominating an individual to be an independent arbiter of quality is also applied to Good Distribution Practices in which a Responsible Person (RP) is introduced in legislation for Pharmaceutical Wholesalers [2].
As explained in Sects. 34.9 and 35.1 the release of pharmaceutical preparations cannot only be based on the assay of the content or on studying the preparation documentation, but has to be based above all on knowledge of and trust of Pharmaceutical Quality Systems. The reporting of incidents, deviations, problems with personnel hygiene and problems with equipment is valuable for a QP, but sometimes there has to be reliance, for those issues, on the head of production. In France the daily physical presence of a QP within a manufacturing plant for radiopharmaceuticals is required before manufacture starts.
The functions of an industrial QP resemble very much the functionality of a quality manager as described in ICH Q10 PQS guidance (see Sect. 35.5.9) or ISO-9001 (see Sect. 35.7.2). It is not surprising that a person fulfilling the QP function is increasingly seen as the quality manager, despite comments that his independent individual opinion may be at stake. The 7 Pillars model (see Sect. 35.7.5) sees the QP as the owner of the “Release System” as indicated by law [4] and as an expert to be consulted about all other elements of the Quality Management System.
All these considerations being true, the very first priorities of a QP position is his autonomy in decision making and in having direct access to senior management.
25.3.4.3 Qualified Person for Pharmacovigilance (QPPV)
The Marketing Authorisation Holder of a product has to have at his disposal a qualified person who is responsible for pharmacovigilance of that product [7]. The MAH is supported by expertise from Regulatory Affairs officers who are responsible for taking care of dossier documentation, including Quality, Toxicology and Clinical Sciences, and updates.
The QPPV should have documented qualifications and experience within pharmacovigilance. If the individual is not medically trained, e.g. a biologist, then the QPPV must have easy access to a medically qualified person. The tasks of the QPPV are extensively described in pharmacovigilance guidelines [3].
Dealing with medical information and complaints of HCP’s and patients is another duty that relates to a potential non-compliance with the marketing authorisation: off label use, medical related complaints.
Each adverse reaction must be investigated and an explanation must be provided whether product quality may be the root cause. Furthermore, it is a GMP requirement that the QP (and delegated QPs) is informed of adverse effects independent of whether these are related to product quality issues.
25.3.4.4 QP/QPPV- Like Functions in Pharmacy
A legal QP and QPPV is required if investigational medicinal products are being prepared for use in clinical trials (see Sect. 35.5.10). Apart from those legally defined functions for the QP/QPPV are not frequently encountered in hospital pharmacies in routine processes but decision making and being held responsible have been assigned to other individuals of which the pharmacist is an example. For example the presence of a QPPV-like function in the hospital pharmacy organisation is required when medicines are imported from outside the EU and are supplied to other pharmacies.
Digifab® Case
After intoxication with digoxin a physician may need digoxin antibodies (Digifab®). Digoxin antibodies bind to digoxin reducing the digoxin level in heart tissue and in blood. This may be a live saving treatment. No licensed product is available in the Netherlands and currently not elsewhere in Europe. However, there is a product on the US market, Digifab®.
In the Netherlands only a few cases per year occur, which does not warrant a stock position in every Dutch hospital. Therefore the Central Hospital Pharmacy in The Hague imports the Digifab® from the USA and serves as a central stock keeper for all Dutch hospitals. An imported medicine is legally considered a non-licensed medicine in the importer country. The Inspectorate agrees with the above construction on conditions:
The request has to be justified and authorised by the treating physician and handed over to the competent authority.
The Inspectorate requires the hospital pharmacy to declare that it performs pharmacovigilance. Therefore the Central Hospital Pharmacy supplies Digifab® together with a request to the physician to report whether or not side effects were observed. Every year a review of all cases is submitted to Inspectorate.
All tasks and responsibilities have to be described in a SOP and Technical Agreement authorised by the relevant involved pharmacists from the distributing pharmacy and the client pharmacies.
25.4 Education
25.4.1 Basic Academic Education
Every pharmacist must be acquainted to the production of medicinal products and product care. So the basic education of pharmacists must include chemical, physical, microbiological and biopharmaceutical disciplines. Knowledge of herbal products and their production must also be a part of the basic pharmaceutical education, as well as pharmacology and toxicology. Directive 2005/36/EC as amended, provides criteria that are applied when a pharmacist from outside the EU can be recognised [9]. This list allows pharmacists to travel and work in the EU countries. It is a minimum list and only applicable for the training of a basic pharmacist. For specialised pharmacists more training is available and in many countries is a requirement. The list has not been based on CanMEDS systematics and is without focus or hints as to the intensity or duration of the training of the subjects of study.
Different types of workers will be employed in pharmaceutical industry with differing levels of education. Many courses are offered to employees both as in-company and courses by outside companies in the educational market. Pharmaceutical manufacturing is a widely used term for these occupations. Pharmaceutical productions operator is more specific for employees with certain well-defined and established jobs in industry. The duration of these courses differ from 20 h to 2 years, depending on the position of the worker and the responsibilities.
As it is assumed that within 5 years knowledge and skills are lost if not applied in practice, initial training at universities should not contain many subjects that are not useful in expected occupations. If a substantial proportion of pharmacists are not involved in production of medicines only the principles of production should be in the training programs. However, this level should allow students to follow specialised pharmaceutical training without delay. This equilibrium has to be maintained by the educational institutes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


