45
Human Immunodeficiency Virus
CHAPTER CONTENTS
Disease
Human immunodeficiency virus (HIV) is the cause of acquired immunodeficiency syndrome (AIDS).
Both HIV-1 and HIV-2 cause AIDS, but HIV-1 is found worldwide, whereas HIV-2 is found primarily in West Africa. This chapter refers to HIV-1 unless otherwise noted.
Important Properties
HIV is one of the two important human T-cell lymphotropic retroviruses (human T-cell leukemia virus is the other). HIV preferentially infects and kills helper (CD4) T lymphocytes, resulting in the loss of cell-mediated immunity and a high probability that the host will develop opportunistic infections. Other cells (e.g., macrophages and monocytes) that have CD4 proteins on their surfaces can be infected also.
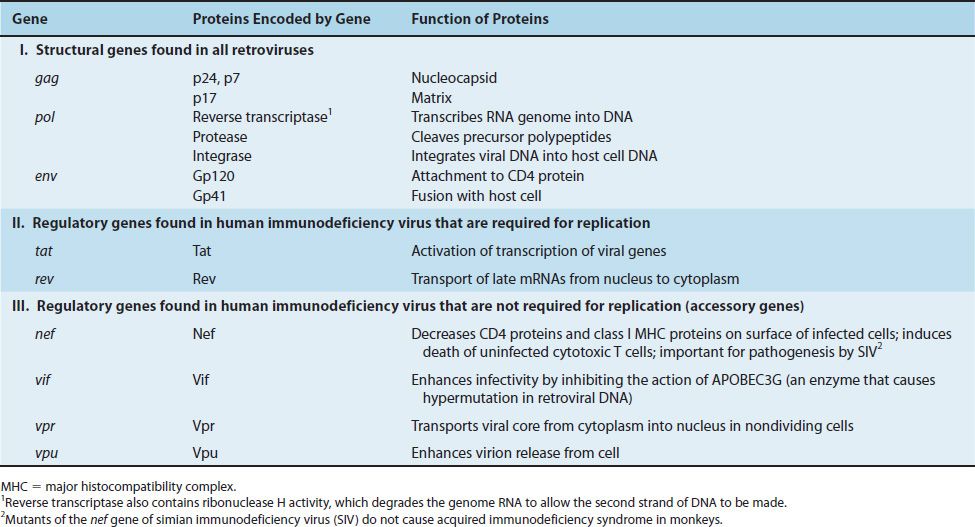
HIV belongs to the lentivirus subgroup of retroviruses, which cause “slow” infections with long incubation periods (see Chapter 44). HIV has a bar-shaped (type D) core surrounded by an envelope containing virus-specific glycoproteins (gp120 and gp41) (Figures 45–1 and 45–2). The genome of HIV consists of two identical molecules of single-stranded, positive-polarity RNA and is said to be diploid. The HIV genome is the most complex of the known retroviruses (Figure 45–3). In addition to the three typical retroviral genes gag, pol, and env, which encode the structural proteins, the genome RNA has six regulatory genes (Table 45–1). Two of these regulatory genes, tat and rev, are required for replication, and the other four, nef, vif, vpr, and vpu, are not required for replication and are termed “accessory” genes.
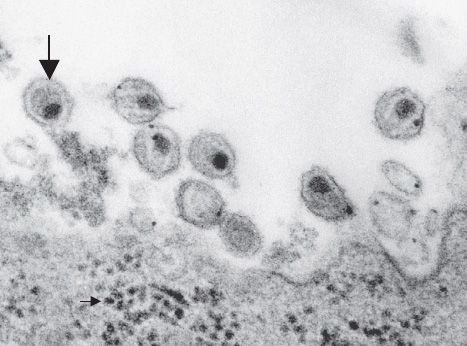
FIGURE 45–1 Human immunodeficiency virus (HIV)—electron micrograph. Large arrow points to a mature virion of HIV that has just been released from the infected lymphocyte at the bottom of the figure. Small arrow (in bottom left of image) points to several nascent virions in the cytoplasm just prior to budding from the cell membrane. (Figure courtesy of Dr. A. Harrison, Dr. P. Feirino, and Dr. E. Palmer, Public Health Image Library, Centers for Disease Control and Prevention.)
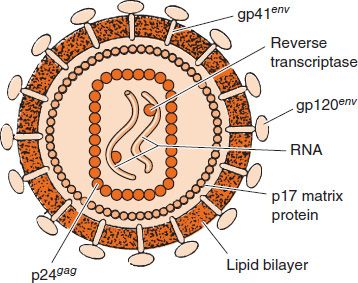
FIGURE 45–2 Cross-section of human immunodeficiency virus (HIV). In the interior, two molecules of viral RNA are shown associated with reverse transcriptase. Surrounding those structures is a rectangular nucleocapsid composed of p24 proteins. Note that the viral protease and integrase are also located within the nucleocapsid (in addition to the reverse transcriptase), but, for lack of space, are not shown in the figure. On the exterior are the two envelope proteins, gp120 and gp41, which are embedded in the lipid bilayer derived from the cell membrane. (Reproduced with permission from Green WC. Mechanisms of disease: the molecular biology of human immunodeficiency virus type I infection. NEJM. 1991;324[5]:309.)
FIGURE 45–3 The genome of human immunodeficiency virus (HIV). Above the line are the three genes for the main structural proteins: (1) gag encodes the internal group-specific antigens (e.g., p24); (2) pol encodes proteins that have four enzymatic activities: protease (PROT), polymerase that functions as a reverse transcriptase (POL), RNase H (H), and integrase (INT); (3) env encodes the two envelope glycoproteins, gp120 and gp41. Below the line are five regulatory proteins: viral infectivity factor (VIF), transactivating protein (TAT), viral protein U (VPU), regulator of expression of virion protein (REV), and negative regulatory factor (NEF). At both ends are long terminal repeats (LTR), which are transcription initiation sites. Within the 5´ LTR is the binding site for the TAT protein, called the transactivation response element (TAR). TAT enhances the initiation and elongation of viral mRNA transcription. (*p24 and other smaller proteins such as p17 and p7 are encoded by the gag gene.)
The gag gene encodes the internal “core” proteins, the most important of which is the p24 protein. It is important medically as it is the antigen in the initial serological test that determines whether the patient has antibody to HIV (i.e., has been infected with HIV) (See “Laboratory Diagnosis” section in this chapter).
The pol gene encodes several proteins, including the virion “reverse transcriptase,” which synthesizes DNA by using the genome RNA as a template, an integrase that integrates the viral DNA into the cellular DNA, and a protease that cleaves the various viral precursor proteins. The env gene encodes gp160, a precursor glycoprotein that is cleaved to form the two envelope (surface) glycoproteins, gp120 and gp41.
Differences in the base sequence of the gp120 gene are used to subdivide HIV into subtypes called clades. Different clades are found in different areas of the world. For example, the B clade is the most common subtype in North America. Subtype B preferentially infects mononuclear cells and appears to be passed readily during anal sex, whereas subtype E preferentially infects female genital tract cells and appears to be passed readily during vaginal sex.
Three enzymes are located within the nucleocapsid of the virion: reverse transcriptase, integrase, and protease (see Figure 45–2).
Reverse transcriptase is the RNA-dependent DNA polymerase that is the source of the family name retroviruses. This enzyme transcribes the RNA genome into the proviral DNA. Reverse transcriptase is a bifunctional enzyme; it also has ribonuclease H activity. Ribonuclease H degrades RNA when it is in the form of an RNA–DNA hybrid molecule. The degradation of the viral RNA genome is an essential step in the synthesis of the double-stranded proviral DNA. Integrase, another important enzyme within the virion, mediates the integration of the proviral DNA into the host cell DNA. The viral protease cleaves the precursor polyproteins into functional viral polypeptides.
One essential regulatory gene is the tat (transactivation of transcription)1 gene, which encodes a protein that enhances viral (and perhaps cellular) gene transcription.
The Tat protein and another HIV-encoded regulatory protein called Nef repress the synthesis of class I major histocompatibility complex (MHC) proteins, thereby reducing the ability of cytotoxic T cells to kill HIV-infected cells. The other essential regulatory gene, rev, controls the passage of late mRNA from the nucleus into the cytoplasm. The function of the four accessory genes is described in Table 45–1.
The accessory protein Vif (viral infectivity) enhances HIV infectivity by inhibiting the action of APOBEC3G, an enzyme that causes hypermutation in retroviral DNA. APOBEC3G is “apolipoprotein B RNA-editing enzyme” that deaminates cytosines in both mRNA and retroviral DNA, thereby inactivating these molecules and reducing infectivity. APOBEC3G is considered to be an important member of the innate host defenses against retroviral infection. HIV defends itself against this innate host defense by producing Vif, which counteracts APOBEC3G, thereby preventing hypermutation from occurring.
There are several important antigens of HIV:
(1) gp120 and gp41 are the type-specific envelope glycoproteins. gp120 protrudes from the surface and interacts with the CD4 receptor (and a second protein, a chemokine receptor) on the cell surface. gp41 is embedded in the envelope and mediates the fusion of the viral envelope with the cell membrane at the time of infection. The gene that encodes gp120 mutates rapidly, resulting in many antigenic variants. The most immunogenic region of gp120 is called the V3 loop; it is one of the sites that varies antigenically to a significant degree. Antibody against gp120 neutralizes the infectivity of HIV, but the rapid appearance of gp120 variants has made production of an effective vaccine difficult. The high mutation rate may be due to lack of an editing function in the reverse transcriptase.
(2) The group-specific antigen, p24, is located in the core and is not known to vary. Antibodies against p24 do not neutralize HIV infectivity but serve as important serologic markers of infection.
The natural host range of HIV is limited to humans, although certain primates can be infected in the laboratory. HIV is not an endogenous virus of humans (i.e., no HIV sequences are found in normal human cell DNA). The origin of HIV and how it entered the human population remains uncertain. There is evidence that chimpanzees living in West Africa were the source of HIV-1. If chimpanzees are the source of HIV in humans, it would be a good example of a virus “jumping the species barrier.”
In addition to HIV-1, two other similar retroviruses are worthy of comment:
(1) Human immunodeficiency virus type 2 (HIV-2) was isolated from AIDS patients in West Africa in 1986. The proteins of HIV-2 are only about 40% identical to those of the original HIV isolates. HIV-2 remains localized primarily to West Africa and is much less transmissible than HIV-1.
(2) Simian immunodeficiency virus (SIV) was isolated from monkeys with an AIDS-like illness. Antibodies in some African women cross-react with SIV. The proteins of SIV resemble those of HIV-2 more closely than they resemble those of the original HIV isolates.
Summary of Replicative Cycle
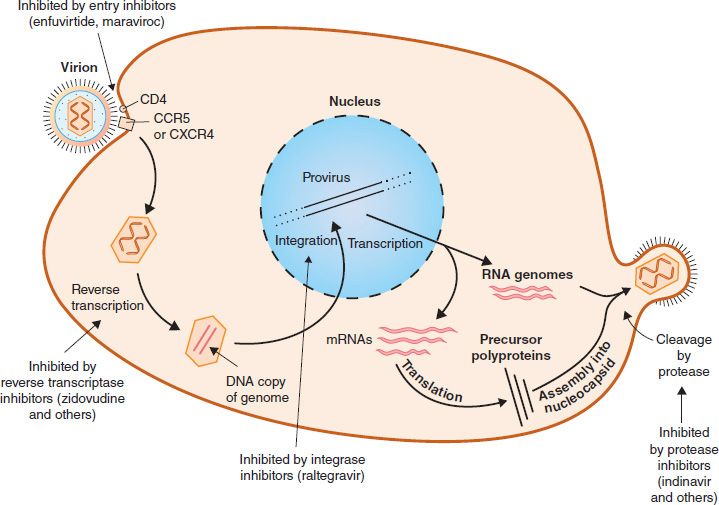
In general, the replication of HIV follows the typical retroviral cycle (Figure 45–4). The initial step in the entry of HIV into the cell is the binding of the virion gp120 envelope protein to the CD4 protein on the cell surface. The virion gp120 protein then interacts with a second protein on the cell surface, one of the chemokine receptors. Next, the virion gp41 protein mediates fusion of the viral envelope with the cell membrane, and the virion core containing the nucleocapsid, RNA genome, and reverse transcriptase enters the cytoplasm.
FIGURE 45–4 Replicative cycle of human immunodeficiency virus (HIV). showing the sites of action of the important drugs used to treat HIV infection. The mode of action of the reverse transcriptase inhibitors, the entry inhibitors, the integrase inhibitor, and the protease inhibitors is described in Chapter 35. On the right side of the figure, “cleavage by protease” describes the process by which the virus-encoded protease cleaves the Gag-Pol polyprotein into functional viral proteins as the virion buds from the cell membrane. These newly formed functional proteins are transported by the mature virion to the next cell and function within that newly infected cell. The viral reverse transcriptase and integrase are two such proteins. (Modified and reproduced with permission from Ryan K et al. Sherris Medical Microbiology. 3rd ed. Originally published by Appleton & Lange. Copyright 1994 McGraw-Hill.)
Chemokine receptors, such as CXCR4 and CCR5 proteins, are required for the entry of HIV into CD4-positive cells. The T cell–tropic strains of HIV bind to CXCR4, whereas the macrophage-tropic strains bind to CCR5. Mutations in the gene encoding CCR5 endow the individual with protection from infection with HIV. People who are homozygotes are completely resistant to infection, and heterozygotes progress to disease more slowly. Approximately 1% of people of Western European ancestry have homozygous mutations in this gene, and about 10% to 15% are heterozygotes. One of the best-characterized mutations is the delta-32 mutation, in which 32 base pairs are deleted from the CCR5 gene.
In the cytoplasm, reverse transcriptase transcribes the genome RNA into double-stranded DNA, which migrates to the nucleus, where it integrates into the host cell DNA. The viral DNA can integrate at different sites in the host cell DNA, and multiple copies of viral DNA can integrate. Integration is mediated by a virus-encoded endonuclease (integrase). Viral mRNA is transcribed from the proviral DNA by host cell RNA polymerase (augmented by virus-encoded Tat protein) and translated into several large polyproteins. The Gag and Pol polyproteins are cleaved by the viral protease, whereas the Env polyprotein is cleaved by a cellular protease.
The Gag polyprotein is cleaved to form the main core protein (p24), the matrix protein (p17), and several smaller proteins. The Pol polyprotein is cleaved to form the reverse transcriptase, integrase, and protease. The immature virion containing the precursor polyproteins forms in the cytoplasm, and cleavage by the viral protease occurs as the immature virion buds from the cell membrane. It is this cleavage process that results in the mature, infectious virion.
Note that HIV replication is dependent on cell proteins as well as viral proteins. First there are the cell proteins required during the early events, namely CD4, and the chemokine receptors, CCR5 and CXCR4. Cell proteins, such as actin and tubulin, are involved with the movement of viral DNA into the nucleus. The cell protein cyclin T1 and the viral protein Tat are part of the complex that transcribes viral mRNA. Cell proteins are also involved in the budding process by which the virus exits the cell.
Transmission & Epidemiology
Transmission of HIV occurs primarily by sexual contact and by transfer of infected blood. Perinatal transmission from infected mother to neonate also occurs, either across the placenta, at birth, or via breast milk. It is estimated that more than 50% of neonatal infections occur at the time of delivery and that the remainder is split roughly equally between transplacental transmission and transmission via breast feeding. There is no evidence for airborne, waterborne, or insect transmission of HIV.
Infection occurs by the transfer of either HIV-infected cells or free HIV (i.e., HIV that is not cell-associated). Although small amounts of virus have been found in other fluids (e.g., saliva and tears), there is no evidence that they play a role in infection. In general, transmission of HIV follows the pattern of hepatitis B virus (HBV), except that HIV infection is much less efficiently transferred (i.e., the dose of HIV required to cause infection is much higher than that of HBV). People with sexually transmitted diseases, especially those with ulcerative lesions such as syphilis, chancroid, and herpes genitalis, have a significantly higher risk of acquiring HIV. Uncircumcised males have a higher risk of acquiring HIV than do circumcised males.
Transmission of HIV via blood transfusion has been greatly reduced by screening donated blood for the presence of antibody to HIV. However, there is a “window” period early in infection when the blood of an infected person can contain HIV but antibodies are not detectable. Blood banks now test for the presence of p24 antigen in an effort to detect blood that contains HIV.
The Centers for Disease Control and Prevention (CDC) estimates that at the end of 2011, there were approximately 1.1 million people infected with HIV living in the United States. The transmission rate has declined markedly, primarily due to increased prevention efforts and improved treatments for HIV; the latter reduces the number of people with high titers of HIV. CDC estimates that approximately 50,000 people new infections occur each year. CDC also estimates that 15% of those who are infected with HIV do not know it because they have not been tested.
Approximately 630,000 people have died of AIDS in the United States since 1981, when AIDS was first recognized.
As of 2011, it is estimated that approximately 34 million people worldwide are infected, two-thirds of whom live in sub-Saharan Africa. Three regions, Africa, Asia, and Latin America, have the highest rates of new infections. AIDS is the fourth leading cause of death worldwide. (Ischemic heart disease, cerebrovascular disease, and acute lower respiratory disease are ranked first, second, and third, respectively.)
In the United States and Europe during the 1980s, HIV infection and AIDS occurred primarily in men who have sex with men (especially those with multiple partners), intravenous drug users, and hemophiliacs. Heterosexual transmission was rare in these regions in the 1980s but is now rising significantly. Heterosexual transmission is the predominant mode of infection in African countries.
Very few health care personnel have been infected despite continuing exposure and needle-stick injuries, supporting the view that the infectious dose of HIV is high. The risk of being infected after percutaneous exposure to HIV-infected blood is estimated to be about 0.3%. The transmission of HIV from health care personnel to patients is exceedingly rare.
Pathogenesis & Immunity
HIV infects helper T cells (CD4-positive cells) and kills them, resulting in suppression of cell-mediated immunity. This predisposes the host to various opportunistic infections and certain cancers such as Kaposi’s sarcoma and lymphoma. HIV does not directly cause these tumors because HIV genes are not found in these cancer cells. The initial infection of the genital tract occurs in dendritic cells that line the mucosa (Langerhans’ cells), after which the local CD4-positive helper T cells become infected. HIV is first found in the blood 4 to 11 days after infection.
HIV infection also targets a subset of CD4-positive cells called Th17 cells. These cells are an important mediator of mucosal immunity, especially in the gastrointestinal tract. Many mucosal Th17 cells are killed early in HIV infection. Th17 cells produce interleukin-17 (IL-17), which attracts neutrophils to the site of bacterial infection. The loss of Th17 cells predisposes HIV-infected individuals to bloodstream infections by bacteria in the normal flora of the colon, such as Escherichia coli.
HIV also infects brain monocytes and macrophages, producing multinucleated giant cells and significant central nervous system symptoms. The fusion of HIV-infected cells in the brain and elsewhere mediated by gp41 is one of the main pathologic findings. The cells recruited into the syncytia ultimately die. The death of HIV-infected cells is also the result of immunologic attack by cytotoxic CD8 lymphocytes. Effectiveness of the cytotoxic T cells may be limited by the ability of the viral Tat and Nef proteins to reduce class I MHC protein synthesis (see later).
Another mechanism hypothesized to explain the death of helper T cells is that HIV acts as a “superantigen,” which indiscriminately activates many helper T cells and leads to their demise. The finding that one member of the retrovirus family, mouse mammary tumor virus, can act as a superantigen lends support to this theory. Superantigens are described in Chapter 58.
Persistent noncytopathic infection of T lymphocytes also occurs. Persistently infected cells continue to produce HIV, which may help sustain the infection in vivo. Lymphoid tissue (e.g., lymph nodes) is the main site of ongoing HIV infection.
A person infected with HIV is considered to be infected for life. This seems likely to be the result of integration of viral DNA into the DNA of infected cells. Although the use of powerful antiviral drugs (see “Treatment” section later) can significantly reduce the amount of HIV being produced, latent infection in CD4-positive cells and in immature thymocytes serves as a continuing source of virus.
Elite controllers are a rare group of HIV-infected people (less than 1% of those infected) who have no detectable HIV in their blood. Their CD4 counts are normal without using antiretroviral drugs. The ability to be an elite controller does not depend on gender, race, or mode of acquisition of the virus. Although the mechanism is unclear, there is evidence that certain HLA alleles are protective and that an inhibitor of the cyclin-dependent kinase known as p21 plays an important role.
In addition, there is a group of HIV-infected individuals who have lived for many years without opportunistic infections and without a reduction in the number of their helper T (CD4) cells. The strain of HIV isolated from these individuals has mutations in the nef gene, indicating the importance of this gene in pathogenesis. The Nef protein decreases class I major histocompatibility complex (MHC) protein synthesis, and the inability of the mutant virus to produce functional Nef protein allows the cytotoxic T cells to retain their activity.
Another explanation why some HIV-infected individuals are long-term “nonprogressors” may lie in their ability to produce large amounts of α-defensins. α-Defensins are a family of positively charged peptides with antibacterial activity that also have antiviral activity. They interfere with HIV binding to the CXCR4 receptor and block entry of the virus into the cell.
In addition to the detrimental effects on T cells, abnormalities of B cells occur. Polyclonal activation of B cells is seen, with resultant high immunoglobulin levels. Autoimmune diseases, such as thrombocytopenia, occur.
The main immune response to HIV infection consists of cytotoxic CD8-positive lymphocytes. These cells respond to the initial infection and control it for many years. Mutants of HIV, especially in the env gene encoding gp120, arise, but new clones of cytotoxic T cells proliferate and control the mutant strain. It is the ultimate failure of these cytotoxic T cells that results in the clinical picture of AIDS. Cytotoxic T cells lose their effectiveness because so many CD4 helper T cells have died; thus the supply of lymphokines, such as interleukin-2 (IL-2), required to activate the cytotoxic T cells is no longer sufficient.
There is evidence that “escape” mutants of HIV are able to proliferate unchecked because the patient has no clone of cytotoxic T cells capable of responding to the mutant strain. Furthermore, mutations in any of the genes encoding class I MHC proteins result in a more rapid progression to clinical AIDS. The mutant class I MHC proteins cannot present HIV epitopes, which results in cytotoxic T cells being incapable of recognizing and destroying HIV-infected cells.
Antibodies against various HIV proteins, such as p24, gp120, and gp41, are produced, but they neutralize the virus poorly in vivo and appear to have little effect on the course of the disease.
HIV has three main mechanisms by which it evades the immune system: (1) integration of viral DNA into host cell DNA, resulting in a persistent infection; (2) a high rate of mutation of the env gene; and (3) the production of the Tat and Nef proteins that downregulate class I MHC proteins required for cytotoxic T cells to recognize and kill HIV-infected cells. The ability of HIV to infect and kill CD4-positive helper T cells further enhances its capacity to avoid destruction by the immune system.
Clinical Findings
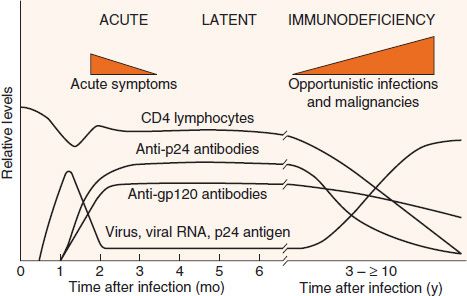
The clinical picture of HIV infection can be divided into three stages: an early, acute stage; a middle, latent stage; and a late, immunodeficiency stage (Figure 45–5). In the acute stage, which usually begins 2 to 4 weeks after infection, a mononucleosis-like picture of fever, lethargy, sore throat, and generalized lymphadenopathy occurs. A maculopapular rash on the trunk, arms, and legs (but sparing the palms and soles) is also seen. Leukopenia occurs, but the number of CD4 cells is usually normal. A high-level viremia typically occurs, and the infection is readily transmissible during this acute stage. This acute stage typically resolves spontaneously in about 2 weeks. Resolution of the acute stage is usually accompanied by a lower level of viremia and a rise in the number of CD8-positive (cytotoxic) T cells directed against HIV.
FIGURE 45–5 Time course of human immunodeficiency virus (HIV) infection. The three main stages of HIV infection—acute, latent, and immunodeficiency—are shown in conjunction with several important laboratory findings. Note that the levels of virus and viral RNA (viral load) are high early in the infection, become low for several years, and then rise during the immunodeficiency stage. The level of CD4 lymphocytes remains more or less normal for many years but then falls. This results in the immunodeficiency stage, which is characterized by opportunistic infections and malignancies. (Reproduced with permission from Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree