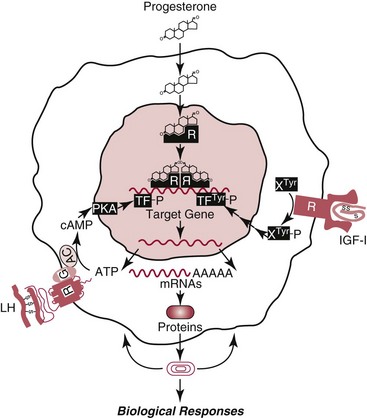
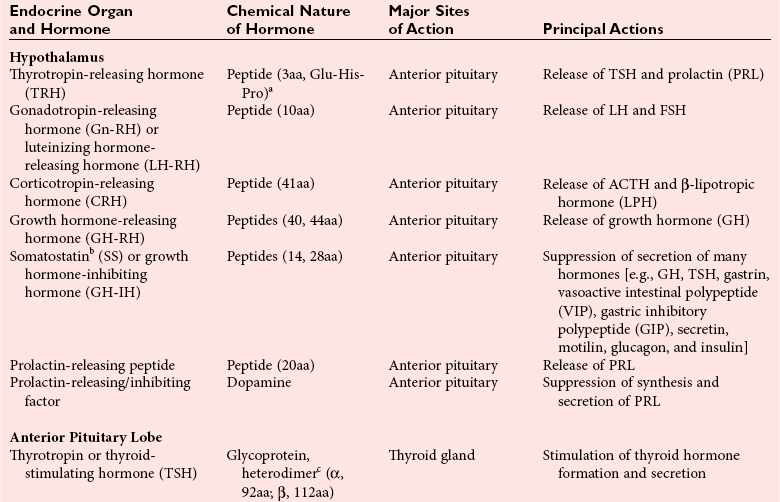
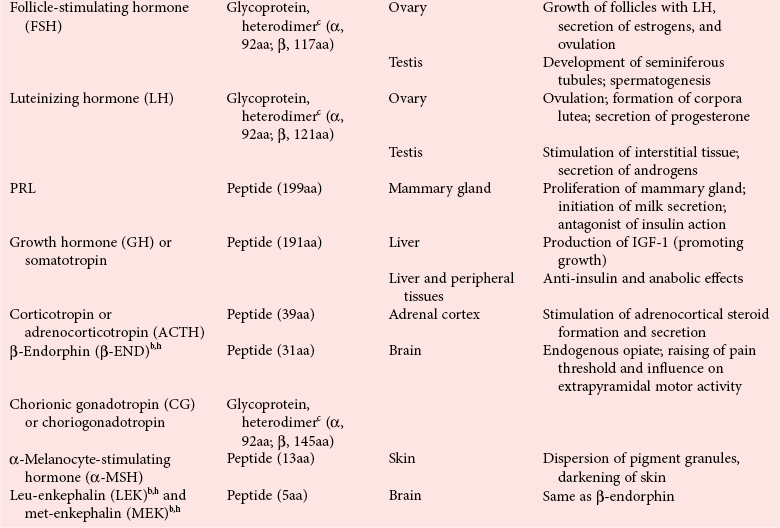
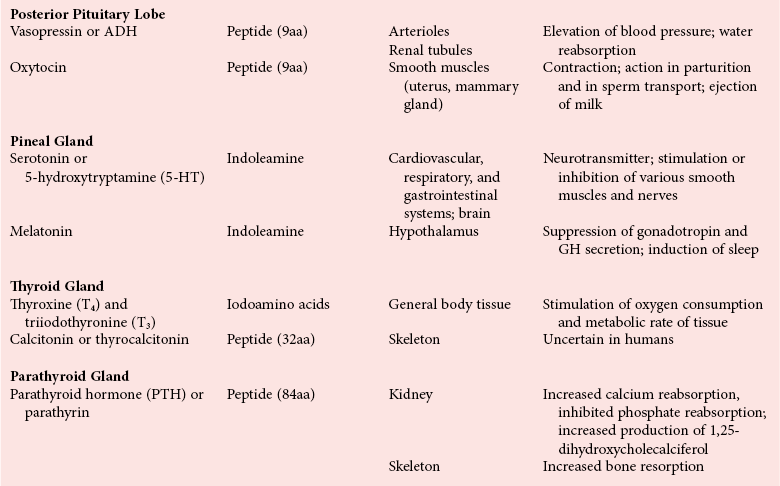
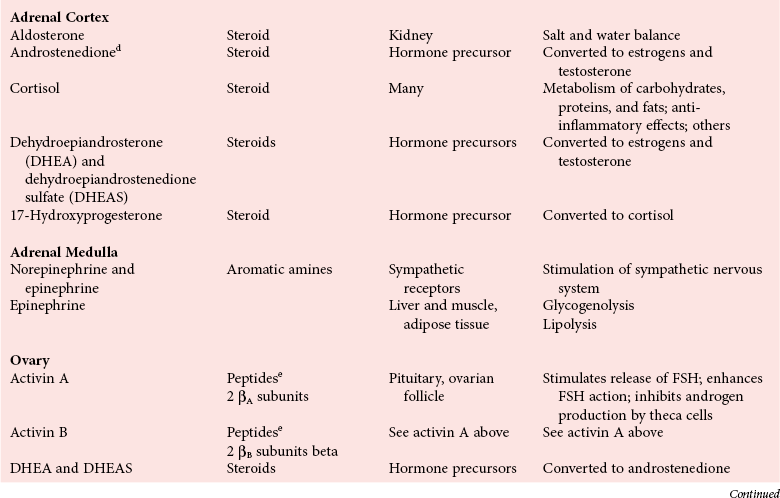
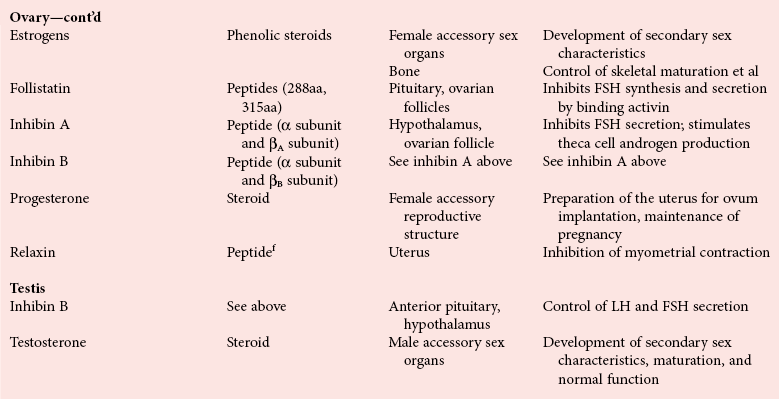
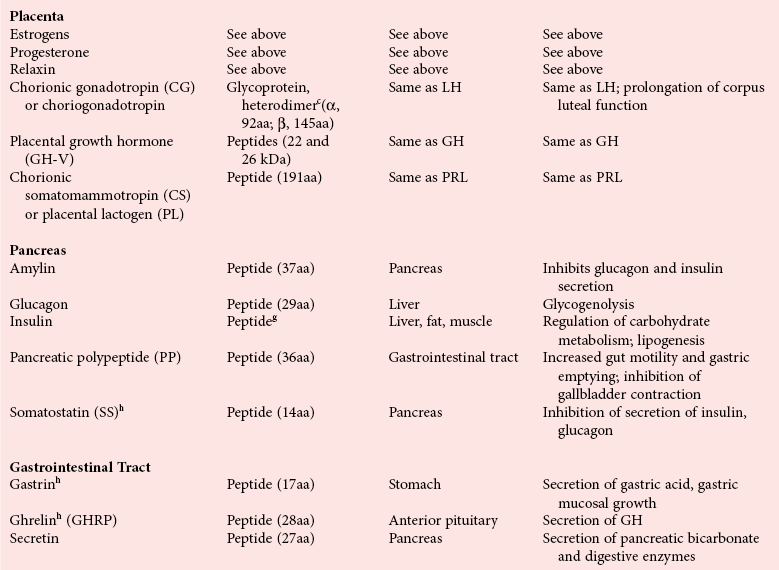
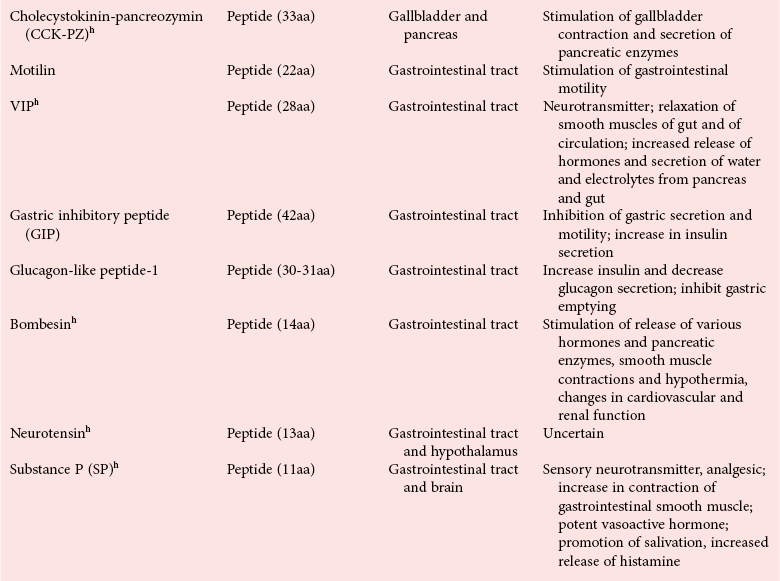
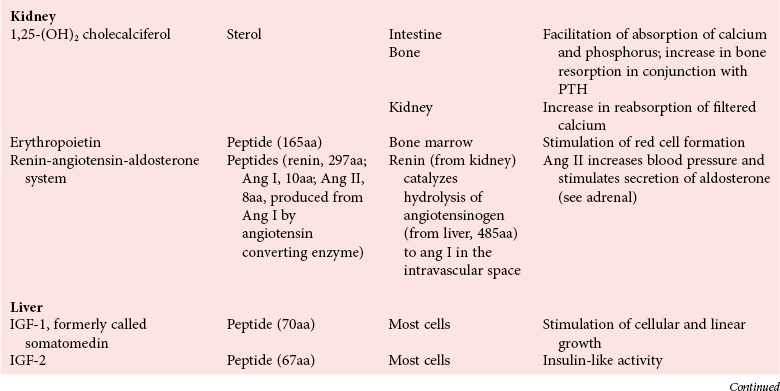
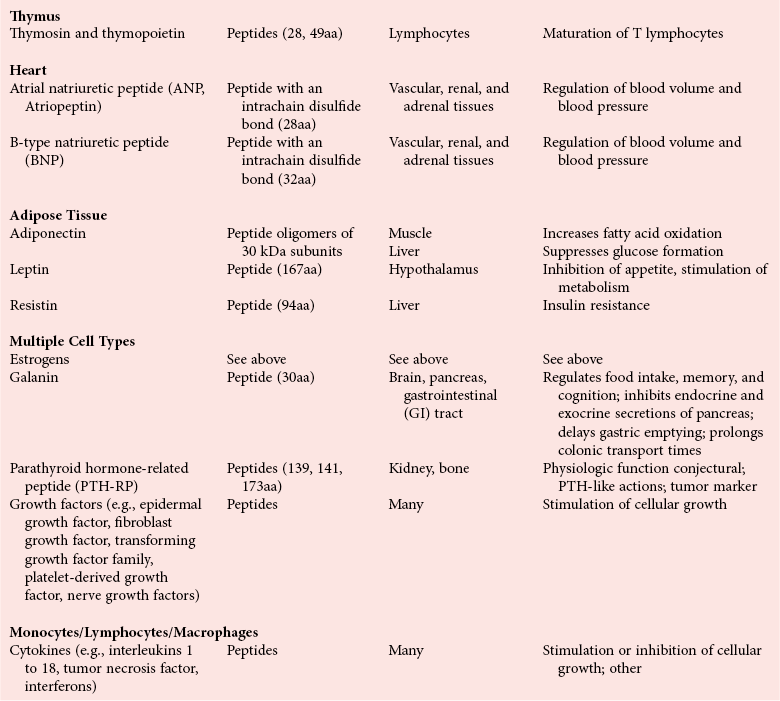
Chapter 29 A hormone is a chemical substance produced in the body by an organ, cells of an organ, or scattered cells, having a specific regulatory effect on the activity of an organ or organs.15 Hormones are produced at one site in the body and exert their action(s) at distant sites through what is called the endocrine system. It is increasingly recognized that many hormones exert actions locally through what is termed the paracrine system. Finally, some hormones exert their action on the cells of origin, regulating their own synthesis and secretion via an autocrine system. The classic endocrine hormones include insulin, thyroxine, and cortisol. Neurotransmitters and neurohormones are examples of the paracrine system, and certain growth factors that stimulate synthesis and secretion of true hormones from the same cell are examples of an autocrine system. Table 29-1 lists hormones that are commonly measured in clinical practice plus a few others to illustrate concepts. Biochemical, clinical, and analytical information for specific hormones may be found in Chapters 26 and 46 through 57. TABLE 29-1 Major Hormones and Frequently Measured Hormone Precursors and Cytokines bAlso produced by gastrointestinal tract and pancreas. cGlycoprotein hormones composed of two dissimilar peptides. The α-chains are similar in structure or identical; the β-chains differ among hormones and confer specificity. dAndrostenedione is also produced in the ovary and testis. eEach activin and inhibin is found in multiple forms. fTwo chains linked by two disulfide bonds: α, 24aa; β, 29aa. gTwo chains linked by two disulfide bonds: α, 21aa; β, 30aa. Steroid hormones (e.g., cortisol, estrogen) are hydrophobic and insoluble in water. These hormones circulate in plasma, reversibly bound to transport proteins (e.g., cortisol-binding globulin, sex hormone-binding globulin) with only a small fraction free, or unbound available to exert physiologic action.5,8,17 The half-life of steroid hormones is 30 to 90 minutes. Free steroid hormones, being hydrophobic, enter the cell by passive diffusion and bind with intracellular receptors in the cytoplasm or the nucleus.3 • Gonadotropins [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)] that regulate the development, growth, and function of the ovary and testis (see Chapter 56). Ovarian and testicular hormones in turn regulate pubertal growth; development and maintenance of secondary sex characteristics; growth, development, and maintenance of the skeleton and muscles; and distribution of body fat. • ACTH that regulates growth of the adrenal glands and synthesis and secretion of adrenal gland hormones (see Chapters 53 and 54). • Thyroid-stimulating hormone (TSH) that regulates growth of the thyroid gland and iodination of amino acids to produce the thyroid hormones triiodothyronine and thyroxine (see Chapter 55).1 • Regulation of blood glucose: In response to a glucose load, insulin is promptly released from the pancreas, which regulates the dispersal of glucose into cells (fat, muscle, liver, and brain) for the metabolism necessary to produce energy from glucose (see Chapter 26). As circulating glucose concentrations thus return to preload concentrations, insulin secretion slows. Several counter-regulatory hormones come into play to further regulate this process to ensure that blood glucose concentrations do not become too low. These include glucagon, cortisol, epinephrine, and growth hormone. Recent attention has focused on a group of gastrointestinal hormones termed incretins (see Chapter 46) that are released during eating and stimulate insulin secretion from the pancreas in advance of any measurable increase in blood glucose. Incretins also affect the rate of absorption of nutrients from the gut by slowing down the rate of gastric emptying. Another mechanism by which incretins have a role in the regulation of blood glucose is by delaying release of the counter-regulatory hormone glucagon from the alpha cells of the pancreatic islets. The most studied incretins are glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP). • Regulation of serum calcium (see Chapter 52): The calcium-sensing receptor (CaSR) on the parathyroid gland recognizes the ambient concentration of ionized calcium, which in turn regulates the synthesis and secretion of PTH. When ionized calcium concentrations fall (so imperceptibly that most analytical methods could not detect the change), PTH synthesis and secretion are stimulated. This additional PTH will attempt to restore serum ionized calcium by enhancing renal tubular reabsorption of calcium and calcium efflux from the skeleton. PTH also catalyzes the synthesis of the renal hormone calcitriol (1,25-dihyroxycholecalciferol), which acts on the gut to increase intestinal absorption of calcium. These very rapid responses of PTH and calcitriol quickly restore ionized calcium to concentrations where the CaSR is no longer activated, and PTH and calcitriol synthesis and secretion return to basal rates. • Water and electrolyte metabolism is regulated by aldosterone from the adrenal gland, renin from the kidney, and vasopressin [antidiuretic hormone (ADH)] from the posterior pituitary gland (see Chapters 48, 53, and 54). The “unique” or specific action of a hormone on its target tissue is a function of the interaction between the hormone and its receptor. As discussed previously, several types of hormone-receptor interactions may occur.3,5,8,17 The hormone-receptor complex provides the very high specificity of the action of the hormone, allowing the target tissue to accumulate the hormone from among all the molecules to which it is exposed. This is essential because hormones generally circulate in picomolar or nanomolar concentrations (10−9 to 10−12 mol/L). Peptide hormones bind to cell surface receptors, and the conformational change resulting from this binding activates an effector system, which in turn is responsible for the downstream actions of the hormone (Figure 29-1).11,12 For most peptide hormones, the intracellular effector that is activated by the hormone-receptor interaction is a specific G-protein (guanyl-nucleotide–binding protein),4,10,13,18 and the receptors are called G-protein–coupled receptors (GPCRs; Figure 29-2). GPCRs are hepta-helical molecules with seven membrane-spanning domains. The amino terminus is extracellular, and the carboxy terminus is intracellular. The major structural classes of GPCRs have been identified, each containing receptors for specific subsets of hormones (Figure 29-3). Group I is the largest group, containing receptors for many peptide hormones and catecholamines. Group II contains receptors for the family of gastrointestinal hormones (secretin, glucagons, and vasoactive intestinal polypeptide). Group III contains the CaSR and the glutamate receptor. Stimulation of a G-protein initiates the intracellular processes of signal transduction that characterize the specific action of the hormone. G-proteins are composed of α, β, and γ subunits and are classified according to the α subunit, of which 20 have been identified to date (see Figure 29-3). G-proteins may stimulate adenylate cyclase (GS type of G-proteins) or may inhibit adenylate cyclase (Gi type). The many classes of GPCRs and G-proteins briefly described in this section provide some insight into the mechanisms responsible for the specificity of hormone action. Some nonpeptide hormones also use cell surface receptors.
Hormones
Classification
Steroid Hormones
Release and Action of Hormones
Growth and Development
Homeostatic Control of Metabolic Pathways
Role of Hormone Receptors
Cell-Surface Receptors
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Hormones