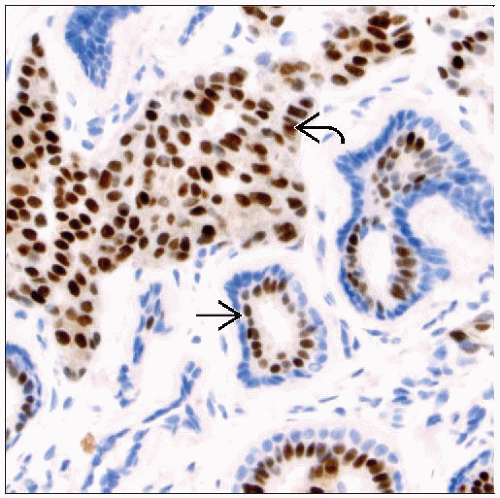
Hormone Receptors (ER/PR)
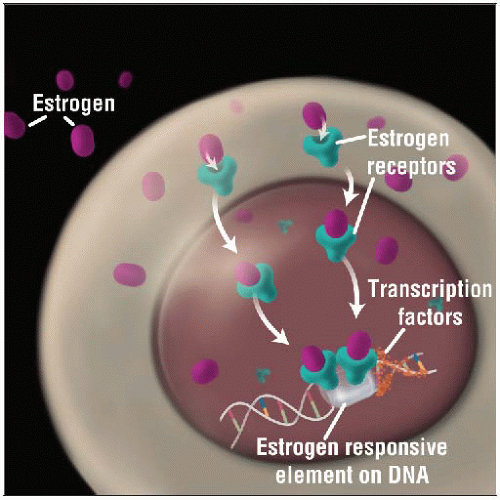 Estrogen receptor binds with its ligand estrogen and is transported to the nucleus of the cell where it acts as a transcription factor, regulating the expression of ER responsive genes. |
TERMINOLOGY
Abbreviations
Estrogen receptor (ER), progesterone receptor (PR)
Synonyms
Estrogen receptor α
Definitions
ER is activated by binding with hormone 17 β-estradiol (estrogen)
ER is found in endometrium, breast, ovarian stroma
2 different forms of estrogen receptor exist: ER-α and ER-β
Each estrogen receptor is encoded by a separate gene: ESR1 (ER-α) and ESR2 (ER-β)
ER-α is most important ER in breast cancer
After binding to its ligand, estrogen, ER is transported to nucleus of cell
In nucleus, ER functions as transcription factor
ER regulates expression of a number of genes important in breast cancer biology
PR expression is regulated by ER
PR is expressed in majority of ER(+) breast carcinomas
EPIDEMIOLOGY
Incidence
ER expression is present in 70-80% of breast cancers
ER(+)/PR(+) ˜ 65%: Usually well- or moderately differentiated cancers
Includes almost all tubular carcinomas, well- or moderately differentiated lobular carcinomas, and mucinous carcinomas
If well-differentiated carcinoma is ER(-), assay may be faulty and should be repeated
ER(+)/PR(-) ˜ 15%: Usually moderately or poorly differentiated; rarely well differentiated
PR is downregulated by HER2; approximately 25% of PR(-) cancers will show HER2 amplification
More frequent in older women with larger cancers with higher rate of proliferation, when compared to ER(+)/PR(+) cancers
ER(-)/PR(+) ˜ 5%: Reported to be more common in younger women with more advanced cancers
Some cases are due to technical problems with either ER assay or PR assay
Biologic basis of PR expression in absence of ER expression is not well understood
ER(-)/PR(-) ˜ 15%: Usually poorly differentiated
About 1/3 of these cancers will show HER2 amplification
These cancers are more common in young women, African-American women, and Latino women
Diet
Cruciferous vegetables (e.g., broccoli, Brussels sprouts) can decrease estrogen exposure
Indole-3-carbinol causes estrogen to be changed to inactive metabolite
Excessive alcohol consumption can increase estrogen exposure
Decreased liver function increases estrogen levels
ETIOLOGY/PATHOGENESIS
Histogenesis
Factors associated with prolonged increased exposure to estrogen are associated with elevated lifetime risk for developing ER(+) breast cancer
Female gender
Early menarche
Late menopause
Obesity after menopause (adipose tissue can be converted into estrogens)
Nulliparity
Hormone replacement therapy
Factors associated with lower estrogen exposure are associated with decreased lifetime risk for developing ER(+) breast cancer
Late menarche
Early menopause
Obesity prior to menopause (menstrual cycles may be reduced or absent)
Child bearing (especially beginning at early age)
Breastfeeding
Oophorectomy
Inappropriate, abnormal, &/or prolonged estrogen exposure stimulates proliferation of ER(+) breast epithelial cells
Increases number of epithelial cells and predisposes cells to mutations, increasing likelihood of ER-dependent breast cancers
CLINICAL IMPLICATIONS
Prognostic Implications
ER expression by breast cancer cells is weak prognostic marker of clinical outcome in most studies
ER expression in breast cancer is highly predictive for clinical benefit from endocrine therapies
Some patients with distant metastases survive for many years with hormonal treatment
In general, ER(+) and HER2(-) carcinomas do not respond well to chemotherapy
PR gene is regulated by ER, and PR is usually detected in tumor cells with activated ER pathway
Recent data has demonstrated that PR status may be independently associated with outcome
ER(+)/PR(+) cancers confer better prognosis than ER(+)/PR(-) cancers
This may be related to different tumor biology for ER(+)/PR(-) subset of cancers
Carcinomas negative for ER and PR have worse prognosis than hormone receptor positive cancers
However, a subset of these cancers (˜ 20%) will have pathologic complete response after chemotherapy, and prognosis for this group is favorable
Patients who develop distant metastases after treatment rarely have prolonged survival
Treatment Implications
ER/PR expression in invasive breast cancer
Clinical benefit from endocrine therapy is only seen in carcinomas that test positive for ER &/or PR
Clinically validated assays for ER and PR should be part of diagnostic work-up of every newly diagnosed invasive breast carcinoma
Endocrine therapy for ER- or PR-positive breast cancer can be achieved using pharmaceuticals or surgery
Drugs
Selective ER modulators (SERMs) act as ER antagonists in breast tissue (e.g., tamoxifen)
Aromatase inhibitors block conversion of precursors to estrogen in peripheral tissue
Gonadotropin-releasing factor can be blocked by antagonists or refractory agonists
Surgery: Ovarian ablation
In patients with BRCA1 or BRCA2 mutations, surgery also reduces risk of ovarian or tubal carcinomas
ER(+)/PR(-) cancers may be more resistant to endocrine therapy
Clinical trial data are unclear as to whether these patients should be treated differently compared to patients with ER(+)/PR(+) cancers
Some medical oncologists are more likely to include chemotherapy in addition to hormonal therapy for ER(+)/PR(-) cancers
ER/PR expression in ductal carcinoma in situ (DCIS)
DCIS typically expresses ER and PR
ER(+)/PR(+) ˜ 85%; ER(-)/PR(-) ˜ 15%; other combinations < 5%
Immunoreactivity can be markedly heterogeneous with both positive and negative areas
May be difficult to distinguish rare tumor cell positivity from residual normal epithelial cells
In majority of cases, ER and PR expression is same for invasive carcinoma and its associated DCIS
In rare cases, expression is discordant (typically DCIS positive and invasive carcinoma negative)
This can lead to inaccurate results for methods that do not distinguish in situ from invasive carcinoma (e.g., some gene profiling assays, automated image analysis)
NSABP B24 DCIS trial had treatment arms with or without tamoxifen; retrospective analysis revealed
Addition of tamoxifen to treatment for DCIS reduced likelihood of recurrence if DCIS was ER(+)
Benefit was not seen for ER(-) DCIS
However, there were too few cases of ER(-) DCIS to exclude possibility of small effect
ER testing of DCIS may be requested by some oncologists to guide treatment decisions
Clinical Assay to Assess ER and PR Status
Currently, ER and PR assessment is performed using IHC techniques
IHC has a number of advantages over ligand-binding assay methodologies
Lower cost
Morphologic confirmation of evaluation of tumor cells and not normal breast elements
IHC detects nuclear hormone receptor proteins and excludes cases with cytoplasmic positivity
Rapid turn around time
Ability to assay smaller tissue samples, such as needle core biopsies