HIV Disease and AIDS
Faith Young Peterson
Key Questions
• What are the common modes of HIV transmission and how can infection be prevented?
• What is the scope of the HIV/AIDS epidemic in the United States and the world?
• How does infection with HIV lead to progressive immunodeficiency and AIDS?
• How has knowledge of the HIV life cycle led to the development of multidrug treatment strategies?
• What are the common systemic manifestations of AIDS and associated opportunistic infections?
• What are the current treatment recommendations for HIV disease and AIDS?
![]()
http://evolve.elsevier.com/Copstead/
This chapter focuses on HIV disease and AIDS—from epidemiology to pathogenesis and management. Human immunodeficiency virus (HIV), an infectious organism, is the prototypical public health infectious disease of the late twentieth century. Originally thought to be a rapid killer, it does not act like other infectious organisms that overwhelm the immune system. HIV infection triggers chronic widespread and diverse organ involvement with varying signs and symptoms. It encompasses all of the armamentarium of a viral infection that has completed the evolutionary progression from animal to human. HIV has done more than just confuse and captivate scientists and health professionals; it also has mobilized risk groups and placed medicine and society at a crossroads of opinion. In this epidemic, the lines between privacy and public health and between morality and compassion have been debated. HIV disease is complex, but in its complexity it has opened the door to better understanding of the immune system.
Human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) are acquired immunodeficiency disorders resulting in defective immune functioning. The hallmark of HIV infection is defective cell-mediated immunity, especially the decrease in CD4+ or T helper/inducer lymphocytes. CD4+ T cells are necessary for appropriate immune responsiveness because they are the cells that mediate between the antigen-presenting cells and other immune cells, such as B cells and other T cells. CD4+ lymphocytes are characterized by the presence of the CD4 receptor.
Epidemiology
HIV infection is a primary immunodeficiency disease caused by the retroviruses HIV type 1 and HIV type 2. Despite research and public health surveillance and prevention activities, the virus has continued mutating and spreading globally. HIV infects people worldwide. However, HIV infection is increasingly becoming a disease of poor, uneducated, or undereducated people of color. Since its identification in the early 1980s, the HIV global epidemic continues with an estimated 33.3 million people living with HIV worldwide as of 2010 and a total of 1.8 million AIDS deaths worldwide in 2009.1 Selected aspects of the global impact of HIV and AIDS are illustrated in Table 12-1.
TABLE 12-1
GLOBAL HEALTH CONSIDERATIONS FOR HIV/AIDS
| COUNTRY/REGION | PREVALENCE OF DISEASE | CULTURAL FACTORS | MOST COMMON MEANS OF TRANSMISSION | TREATMENT | ECONOMIC/SOCIAL IMPACT |
| Sub-Saharan Africa∗ | 22.5 million people affected. This region carries 70% of the world’s HIV/AIDS burden Adult prevalence % = 5.0% Adult and child deaths due to AIDS = 1.3 million | Gender inequalities: Males dominate sexual decision making and women are disproportionately infected | Heterosexual sex and mother-to-child transmission | Treatment available, but not affordable for most people Treatment coverage: 37% (3,911,000) | Life expectancy has decreased dramatically and population is beginning to bottleneck Reversing the progress in poverty reduction Stigma associated with disease causes people to lose property |
| East Asia/China† | 770,000 people affected Adult prevalence % = 0.1% Adult and child deaths due to AIDS = 36,000 | Growing male demographic has led to a growing sex industry | Intravenous drug use, prostitution, and transmission through migrant workers | Medical coverage in rural areas is poor, so a large proportion of the population must pay out of pocket. Treatment coverage: 31% | Cost of therapy generally exceeds annual income Stigma is so great that many people do not disclose their HIV status to their families and thus do not seek treatment |
| North America | 1.5 million people affected Adult prevalence % = 0.5% | Continued stigma | Men having sex with men (MSM), intravenous drug use, heterosexual transmission and prostitution | Treatment available to most people | Cost of therapy is high but programs are available for assistance |
| Western and Central Europe | 820,000 people affected Adult prevalence % = 0.2% Adult and child deaths due to AIDS = 76,000 | Continued stigma | MSM, intravenous drug use, heterosexual transmission and prostitution | Treatment available to most people | |
| South and Southeast Asia | 4.1 million people affected Adult prevalence % = 0.3% Adult and child deaths due to AIDS =260,000 | Stigma Drug use and prostitution high in some areas such as Thailand | MSM, intravenous drug use, prostitution | Cambodia and Thailand have 50-80% antiretroviral coverage | |
| Central and South America | 1.4 million people affected Adult prevalence % = 0.5% Adult and child deaths due to AIDS = 58,000 | Men who have sex with men are highly stigmatized, so prevention efforts overlook this group. Drug use is commonplace | MSM, intravenous drug use, and prostitution | 50-80% of infected people are receiving antiretroviral therapy. The government provides therapy for free. | Government has had a strong and positive response to the epidemic: Its efforts have reduced stigma, improved social reintegration, and reduced HIV prevalence among high risk populations. |
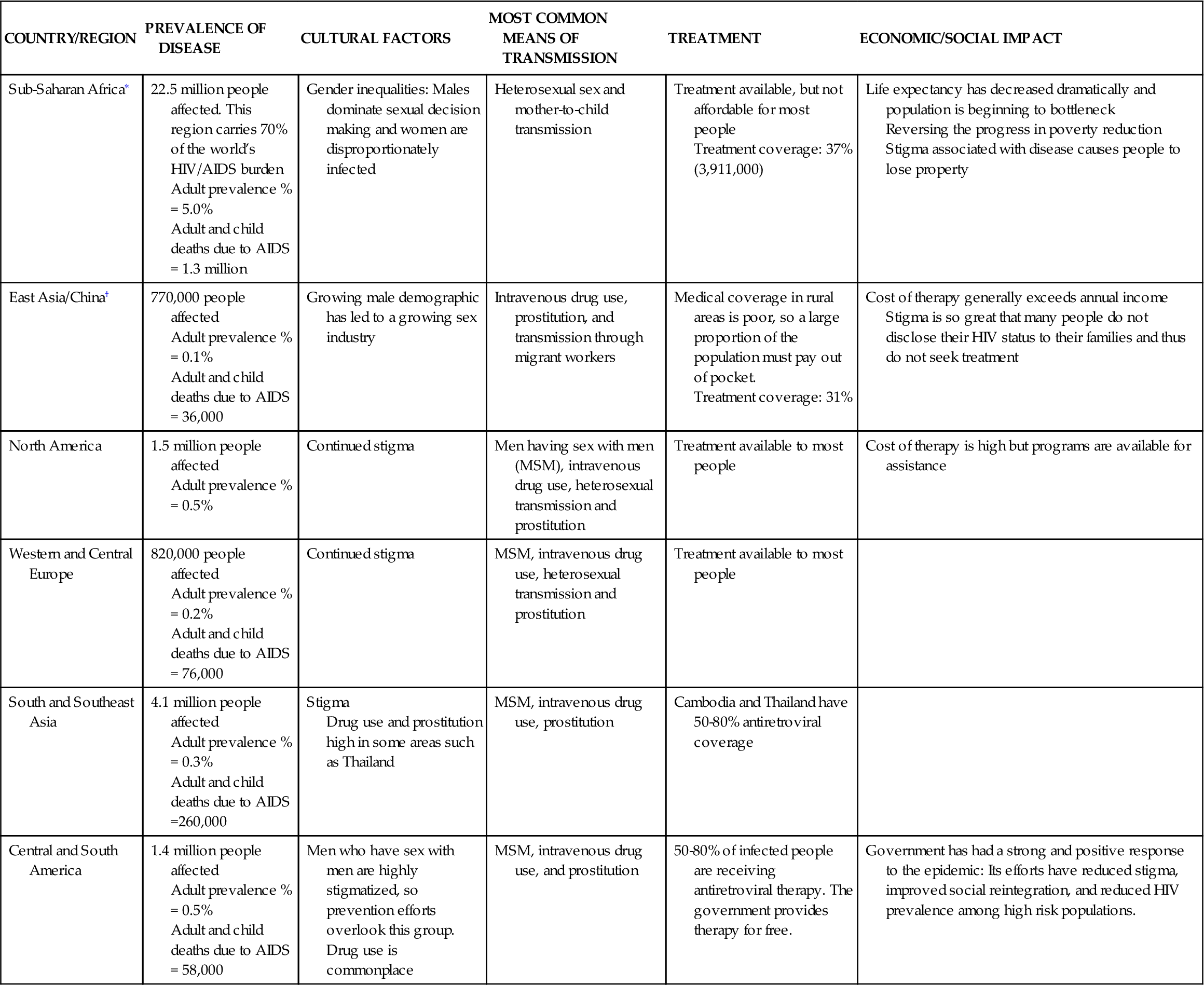
∗USAID (2010). HIV/AIDS health profile. USAID From the American People. Retrieved from http://www.usaid.gov/our_work/global_health/aids/Countries/lac/brazil.pdf.
The total adult prevalence is now 0.8% of the adult population of the world.1 Of the 33.3 million people infected with HIV, women comprise 15.9 million and children <15 years old comprise 2.5 million.1 According to the World Health Organization (WHO), the number of new HIV infections globally declined 19% over the past decade, attributable to expanded and improved HIV programs.1 However, HIV infection rates continue to increase in sub-Saharan Africa as well as in eastern Europe and central Asia. In third world countries, those infected with HIV have more limited access to testing and medication for treatment, as well as limited information for prevention because of the effects of gender inequity and harmful social norms that drive transmission.1 For example, only 37% of those infected in sub-Saharan Africa receive antiretroviral therapy compared to 50% in Latin America and the Caribbean.1
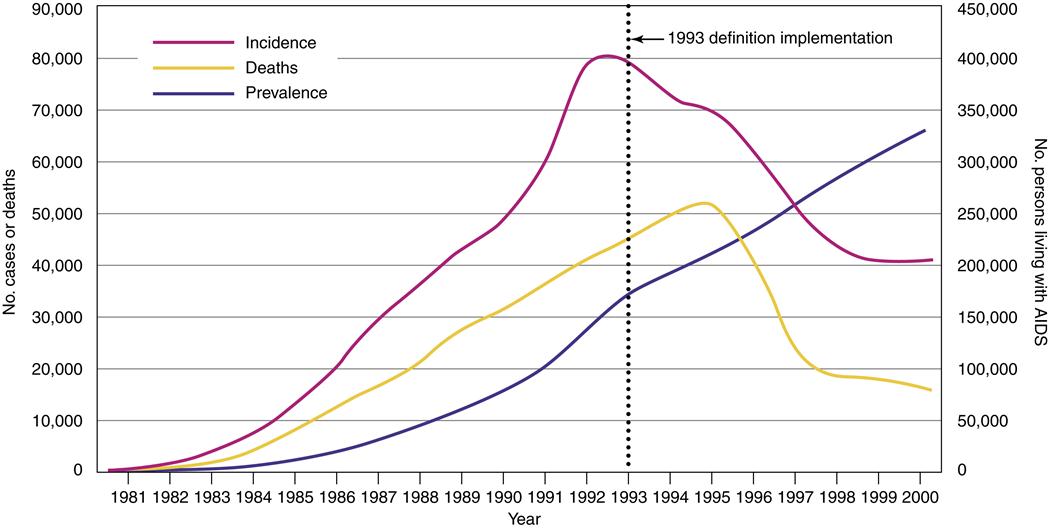
In the United States, more than 1 million people have been diagnosed with HIV/AIDS since the beginning of the epidemic.2 It is estimated by the Centers for Disease Control and Prevention (CDC) that there are 663,084 persons living with HIV/AIDS in the United States, with 56,000 new HIV infections diagnosed yearly.2 In the United States and other industrialized countries where access to medication, care, and prevention is greater, the number of patients diagnosed with and dying from AIDS is stable or declining (Figure 12-1). The proportion of people living 3 or more years after an AIDS diagnosis in the United States has increased. However, the CDC estimates that up to 25% of the people who are infected with HIV in the United States may be unaware that they are infected.
Current statistics show that of the people diagnosed with HIV/AIDS in the United States, racial and ethnic minorities, women of color, and men who have sex with men (MSM) are disproportionately affected.2 Overall, there has been a decrease in the rates of new diagnoses between 1998 and 2007 by 20.7%—from 18.4 to 14.6 new cases per 100,000 population.3 Among racial and ethnic groups, most of the new cases are in black non-Hispanics, followed by Hispanics.3–5 Males had a higher rate of diagnoses (21.9 per 100,000 in 2007) than females (7.6 per 100,000 population).3 According to the CDC, 75% of new HIV infections occur in men and of those 50% occur in men who have sex with men (MSM).4 The highest rates of new diagnoses of HIV are in black men and women, with a lifetime risk of 1 in 16 for black males and 1 in 30 for black females compared to 1 in 104 for white males and 1 in 588 for white females.5 The rate of diagnoses also varied by state, with the District of Columbia having 154.6 new cases per 100,000 population, which is the highest rate in the country.3 It is thought that noninjection drugs (such as crack cocaine or methamphetamine) may also contribute to the spread of HIV/AIDS because of sex trading for drugs, shelter, or money. Ninety percent of children younger than age 13 are infected perinatally.6 Ten to eleven percent of HIV cases are in people older than age 50 often as a result of a decreased perception of risk. As a result, older adults are often diagnosed later with a death rate that is higher than in other age groups.
History
In 1981 the first descriptions of immunodeficiency disease in previously healthy persons appeared in the medical literature. At that time, previously healthy young homosexual men in increasing numbers contracted unusual diseases for their age group, such as Pneumocystis jiroveci (carinii) pneumonia (PCP) and Kaposi sarcoma, that researchers identified as HIV. The first evidence of alternative forms of transmission of the virus by blood and blood products appeared in 1982. All these early patients were shown to have a type of HIV virus called HIV-1. It was at this time that the term acquired immunodeficiency syndrome (AIDS) was first used. However, the specific retrovirus causing HIV infection and AIDS was not isolated until the early to mid-1980s. The timeline of HIV history is found in Table 12-2.
TABLE 12-2
| 1900 | Retroviruses identified as cause of cancer in chickens |
| 1930s | HIV-1 precursor virus crossed species from chimpanzees to humans during hunting from contact with infected chimpanzee blood |
| 1950s | Positive tests on serum from man in Leopoldville (now Kinshasa) Congo |
| 1960s | Virus reaches Haiti |
| 1968 | First case in U.S. in sexually active 15-year-old African-American male in St. Louis |
| 1980 | First retrovirus identified in humans affecting T cells (human T cell lymphocytic virus, HTLV-1) |
| 1981 | Syndrome of HIV first reported in healthy young homosexual men in LA and New York |
| 1982 | Identification of HIV transmission by blood/blood products with first use of term AIDS Syndrome also identified in women, infants, Haitians, and persons who had received blood or blood products |
| 1983 | CDC publishes first Blood and Body Fluid Precautions First WHO meeting on AIDS |
| 1985 | First HIV-1 antibody testing (ELISA and Western Blot) First international conference on AIDS |
| 1987 | HIV-2 identified in visitor to U.S. from West Africa First anti-HIV drug approved CDC revises guidelines with identification of mucocutaneous exposure – “Universal Blood and Body Fluid Precautions” |
| 1988 | World Health Organization (WHO) declares December 1 as World AIDS Day |
| 1994 | CDC announces that AIDS is leading cause of death among Americans 25 to 44 years old |
| 1996 | Combination antiretroviral therapy (HAART) is introduced |
| 1999 | Researchers discover origins of HIV-1 from chimpanzee species (see 1930s) |
| 2006 | WHO declares March 8 as National Women’s & Girls HIV/AIDS Awareness Day SMART trial found that episodic antiretroviral therapy more than doubles risk of AIDS or death in people with HIV infection |
Types of HIV
HIV is a type of retrovirus from the subfamily Lentivirinae, with Lentivirus being its only genus. This subfamily is so named from the Latin word lentus, meaning “slow,” because infection develops gradually. HIV-2, a related but distinct retrovirus, was later identified in 1986 and is most closely related to simian immunodeficiency virus. HIV-2 is differentiated from HIV-1 by a longer clinical latency period from the onset of infection to the development of symptoms. It is also characterized by having lower plasma HIV-2 viral loads and lower mortality rates and by generally being a milder form of the disease.6 HIV-2 infection can progress to AIDS, even though it appears to be less virulent than HIV-1. It is also possible to be coinfected with both HIV-1 and HIV-2.6
Both HIV-1 and HIV-2 are found worldwide. They are similar in structure and function but are differentiated from each other by their envelope glycoproteins, point of origin, and latency periods. The point of origin for HIV-1 is Central Africa and for HIV-2 it is West Africa. HIV-1 is the causative organism of most cases found in Central Africa, the United States, Europe, and Australia. HIV-2 is found primarily in West Africa or in countries with strong socioeconomic ties to West Africa (e.g., France, Spain, Portugal, and former Portuguese colonies).6
Many subspecies or strains of HIV also exist because of the rapid rate of HIV virion mutation. The subspecies may exist in different hosts, as well as within an individual host. Currently, at least 10 subtypes of HIV-1 have been identified: group N (YBF30), group O, and group M with 8 subtypes (A, B, C, D, E, F, G, H). Research is currently focusing on the identification of HIV subtypes and strains in different populations and geographic areas. For example, in the United States, Europe, and Australia, most infected persons have HIV-1, subtype B, whereas in India, HIV-2 is found near Goa, and HIV-1 strains A, B, and C are also present.6
Transmission
HIV-1 and HIV-2 are relatively weak viruses outside of the body. HIV viruses can infect people through three major types of transmission: sexual transmission via semen or vaginal and cervical secretions through homosexual, bisexual, or heterosexual intercourse; parenteral transmission via blood, blood products, or blood-contaminated needles or syringes; and perinatal transmission in utero, during delivery, or in breast milk. Of these forms of transmission, sexual transmission through unprotected vaginal or anal intercourse is the most common mode of infection globally. In very low titers, HIV is known to be present but has not been shown to be transmitted via urine, saliva, tears, cerebrospinal fluid, amniotic fluid, and feces. HIV is not known to be transmitted via aerosol routes. In the United States, those at greatest risk of HIV infection include (1) men having sex with men (MSM); (2) intravenous drug users (IVDs) who share needles or syringes; (3) sexual partners of those in high-risk groups, particularly heterosexual women; and (4) infants born to infected mothers. Heterosexual intercourse with infected partners, contact with contaminated blood, and prenatal or perinatal exposure of the infant prenatally are the major routes of transmission of HIV in Africa, South and Southeast Asia, and developing countries.1 In these countries, an equal proportion of males and females are infected.
Common modes of transmission include needle/syringe sharing between intravenous drug users, unprotected sex with infected partners, recipients of HIV-contaminated blood or blood products or infected semen during artificial insemination, unanticipated needle or scalpel injury during care or surgical treatment of infected patients, and neonatal transmission from an infected mother to her infant. In both men and women, concomitant sexually transmitted diseases or genital lesions increase the risk of HIV infection. In women, high-risk heterosexual contact is influenced by lack of HIV knowledge, low socioeconomic status, low perception of risk, concomitant drug or alcohol use, relationship dynamics such as fear of abuse or loss of relationship, and the increased biological vulnerability of HIV contraction during vaginal intercourse, especially in the presence of other sexually transmitted diseases or vaginal inflammation. In both men and women, the use of noninjection drugs (such as crack cocaine) contributes to HIV transmission by decreasing inhibition, allowing the person to engage in risky sexual behaviors or to trade sexual relations for drugs, money, or shelter.
Blood bank screening and testing procedures have nearly eliminated the transmission of HIV-contaminated blood in the United States. In the United States, all blood and blood products have been tested by HIV-1, HIV-2, and HIV-1 p24 antigen tests since 1996. However, this route of transmission continues in third world countries, where there is a high number of HIV-infected persons and much of the blood and blood products are not screened before use.
Health care workers who are exposed to blood or infected body fluids or needles/sharp instruments are at risk of contracting HIV. The risk of developing HIV is greatest for those health care workers who have a deep injury with visible blood from a contaminated needle or sharp instrument or who have a direct puncture into an artery or vein. They also are at risk if they have prolonged blood-skin contact, especially if extensive. The risk of infection is much lower when both universal precautions and postexposure prophylaxis are employed.
Transmission from an infected mother to her infant may occur in the intrauterine period, in the intrapartum period at the time of delivery, or in the postpartum period via breast feeding; it may also be transmitted in some cultures from saliva attributable to premastication. Of these, intrapartum transmission at the time of delivery is thought to be the most common. HIV infection does not cause any specific congenital abnormalities, but there is an increased risk of spontaneous abortion. The overall risk to the fetus of HIV transmission is estimated to be between 15% and 40% for each pregnancy, with increasing risk in subsequent pregnancies for each HIV-positive fetus born. Increased risks of antepartum transmission include increased maternal viral load or high viremia during early infection, advanced maternal clinical disease as evidenced by low CD4+ counts, and breaks in the placental barrier. Increased risks of intrapartum transmission include high maternal viral load at the time of delivery, prolonged ruptured membranes (more than 4 hours), infant exposure to blood/secretions, abruptio placentae, infant prematurity, and the presence of coinfections. The rate of HIV perinatal transmission is reduced with the use of antiretroviral therapy during pregnancy and during the first months of the infant’s life.
Routine social contact with people who are HIV positive does not increase one’s risk of HIV infection. The following examples are safe practices and will not cause exposure to HIV infection: using public restrooms, swimming in public swimming pools, touching or hugging someone who is HIV positive, and eating with community utensils or in restaurants. Insects such as mosquitoes cannot transmit the HIV virus to humans.
Exposure to HIV does not mean that one will contract HIV or AIDS, and it does not mean rapid progression. The interacting forces between viral and host factors influence whether a person will contract HIV infection, particularly the amount and virulence of the virus and the host’s response by T cell–mediated cytotoxicity or by cytokines. For example, a woman’s plasma viral load may predict the amount of her genital HIV viral shedding, which may influence the exposure to HIV virion by her sexual partner.7 In studies of patients with hemophilia who received tainted blood products, 10% to 25% of the individuals evaded infection. Because of genetic differences that either increase or decrease susceptibility to the infection, the risk of acquiring HIV and the response to infection also vary within populations.8 Despite infection for more than 10 years, some infected individuals remain symptom free; and some individuals, despite high-risk exposure, do not exhibit any signs of infection or immunodeficiency.8
Researchers have identified an HIV resistance mutation of the CCR5 gene, called CCR5-delta 32, which is associated with natural resistance to HIV infection in certain people.8 When inherited from both parents, the mutant CCR5-delta 32 gene appears to protect individuals from infection even after multiple exposures. When only one gene is inherited, the progression to AIDS tends to be slower.8 The CCR5 gene is not equally distributed among people. Persons of Caucasian-American and Caucasian-European descent have the highest number of mutant allele genes, approximately 10%, while Native American, African, and East Asian people have the lowest number of mutant alleles.8 Researchers have also found that other mutations in the CCR5 gene can delay progression of HIV infection, such as polymorphism-2459 (A/G).8 There are other proteins that bind to CCR5 and demonstrate antiviral activity, such as macrophage inflammatory protein-1α (MIP-1α) and MIP-β, as well as chemokines and other factors such as stromal-derived factor (also known as pre-B cell growth–stimulating factor).9 These factors can also effectively block HIV-1 infection in some people. People who have fewer genes encoding CCL3L1, a potent HIV-blocking protein that interacts with CCR5, are more susceptible to HIV infection and have more rapid progression to AIDS whereas individuals with natural killer (NK) cells that produce interferon-γ, tumor necrosis factor-α, CCL3, CCL4, and CCL5 are less likely to develop HIV infection.8–10
Prevention of Transmission
Prevention is essential, because effective management of HIV is expensive and a cure is not yet possible. However, one-time exposure to information or a single message is usually less successful than programs that teach prevention skills and reinforce positive behavior. The primary way to prevent transmission is to use safe sex practices. Safe sex practices include abstaining from sex, using a condom (barrier protection) during sexual intercourse, avoiding multiple sexual partners, and knowing the HIV status of all sexual partners. It is important that education regarding safe sex practices be tailored to appropriate age groups, ethnicity, culture, and sexual preference. Patient visits to health care providers are an excellent opportunity to encourage individual HIV protection.
Spermicides such as nonoxynol 9 or C31G do not inactivate HIV or other sexually transmitted microorganisms. No studies suggest any benefit from using progestins such as levonorgestrel (Norplant) or medroxyprogesterone (Depo-Provera), the diaphragm, or oral contraceptives to prevent HIV transmission. The early use of antepartum and intrapartum antiretroviral therapy and avoidance of breast feeding can prevent maternal-child HIV infection.
HIV infection in drug users can be prevented with the use of sterile needles via improved access to clean needles and avoidance of dirty or shared needles. Such intervention includes needle/syringe exchange programs for IVDs and cleaning of dirty needles with bleach before use. When using bleach, the user must rinse out all blood first; then fill the needle and syringe with full-strength bleach at least three times for 30 to 60 seconds.
Medical and health care personnel are at risk through occupational exposure to blood and body fluids. Self-protection through the use of standard precautions can decrease risk by reducing exposure. Health care providers should carefully wash their hands before and immediately after patient contact even when using gloves. It is essential to wear disposable gloves for any actual or potential contact with blood or body secretions, when handling items contaminated with blood or body fluids, when performing finger sticks or heel sticks, or when the health care provider has scratches or cuts on the hand.
Gowns or plastic aprons, masks, goggles, or face shields should be worn to protect the face and clothing when there is risk of splashes and airborne droplets of blood or body fluids. Protective gear should be changed between patients. Careful prevention of parenteral exposure when using needles or other equipment should be emphasized. Needles and sharp implements should be disposed in rigid, puncture-proof containers. Such implements should not be bent, broken, or recapped before disposal. In combative patients who must have blood drawn or injections given, careful use of humane and limited restraint devices may be necessary to prevent injury to the involved health care workers. Resuscitation bags and masks should be readily available to minimize the need for mouth-to-mouth procedures.
Unfortunately, accidents necessitating the development of postexposure prevention protocols do occur. If a health care worker sustains an injury with significant exposure to HIV-infected blood or body fluids such as a needle stick, a workplace postexposure prevention protocol should be followed immediately. According to the U.S. Public Health Service and National Institutes of Health (NIH), the selection of a drug regimen for HIV postexposure prophylaxis must balance the risk for infection against potential toxicities and side effects of the medication(s). As such, consultation with an infectious disease provider is recommended. Treatment usually depends on knowledge of the viral status and/or viral load of the exposure source; this information will help determine the appropriate antiretroviral therapy regimen that should be implemented. Often postexposure protocols involve the administration of two or three medications. The length of administration of the agents depends on multiple factors and may be 4 weeks or longer. This same protocol has been advocated for use as post-sexual exposure prophylaxis.
Etiology
HIV Structure
HIV is an RNA retrovirus that causes a defect in cell-mediated immunity that may progress to AIDS. The viral RNA must be converted to DNA before the viral genes can be expressed to make copies of the RNA virus. Like other retroviruses, HIV differs from DNA viruses in that the RNA genome cannot replicate without undergoing conversion into DNA.
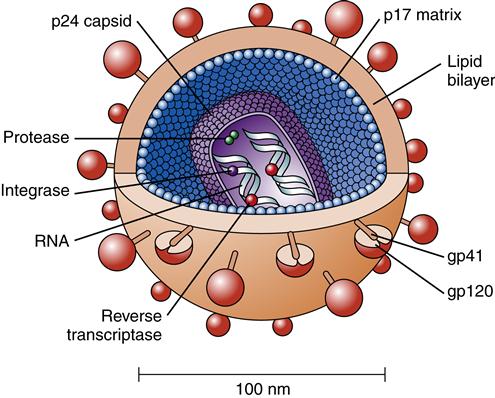
HIV consists of a core or nucleocapsid containing two strands or chains of RNA, protein, and enzymes surrounded and protected by a spherical lipid bilayer viral envelope that is 0.0001 mm in diameter. Between the envelope and core is a protein layer called p17. The nucleocapsid or core is composed of a protein called p24. Within the nucleocapsid, the two strands of RNA compose the HIV genome (Figure 12-2). The HIV genome consists of at least nine genes. The gag gene encodes the core antigen proteins. The pol gene encodes reverse transcriptase proteins. The env gene encodes the viral envelope protein glycoprotein gp160, which is split into two fragments, gp120 and gp41, by cellular protease.
Several other genes have been identified, including tat, rev, nef, vif, vpr, and vpu. These genes are primarily regulatory genes. The tat gene encodes proteins that regulate HIV replication and can accelerate HIV viral protein production. It is controlled by tat-binding protein. The rev gene encodes proteins that regulate viral messenger RNA expression. Rev proteins inhibit regulatory proteins, allowing the transport of HIV RNA from the nucleus. Rev proteins also enhance viral structural gene production. The vif (virion infectivity factor) gene appears to increase the ability of the virus to infect other cells. It suppresses the human protein (CEM 15) that inhibits HIV-1.
The HIV genome contains all the information regulating the virus’s structural format and growth during its life cycle. The enzymes within the core also are very important because they facilitate the conversion of RNA to DNA. This conversion is the means of information transfer. The enzymes include reverse transcriptase, integrase, and protease. Reverse transcriptase is composed of two associated enzymes called polymerase and ribonuclease. It is the unique enzyme in HIV that allows the virus to copy RNA into DNA. Protease is a complex enzyme that works as a “molecular scissors.” It splits the other viral components by a process known as autocatalysis. Immature, noninfectious virions containing inactive gag/pol, a long precursor protein, are released in the plasma, where they are cleaved by protease into smaller active units. Protease also clips p55, the core gag viral protein precursor, into smaller molecules and is needed to facilitate final mature viral assembly for HIV to be infectious.11 In other words, HIV infection does not occur unless protease activates the virions.
The viral envelope consists of a membrane derived from the host cell. Viral glycoprotein studs protruding from the cell membrane make it look like a studded ball (Figure 12-3). Gp120 and gp41 are the two HIV envelope proteins that cover the viral particle surface. Gp120 is the most external and distal part of each “stud,” whereas gp41 is the bridge that holds it onto the virion surface. The surface envelope also contains other cell surface proteins derived from the host cell containing adhesion molecules. Although the viral particle (virion) is nearly spherical, great diversity is found in size and shape, such as comet-shaped virions and virions with tails.
HIV Binding and Infection
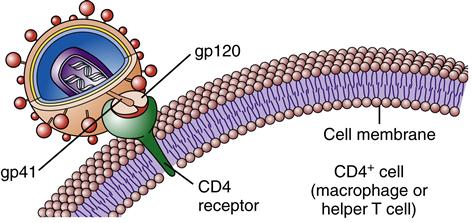
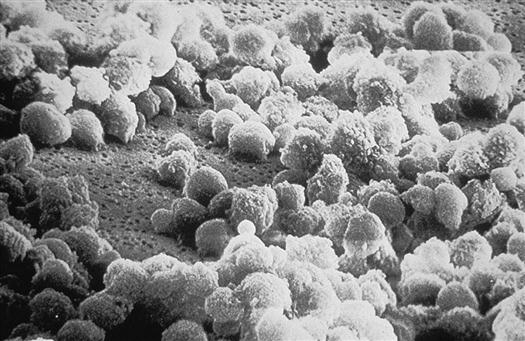
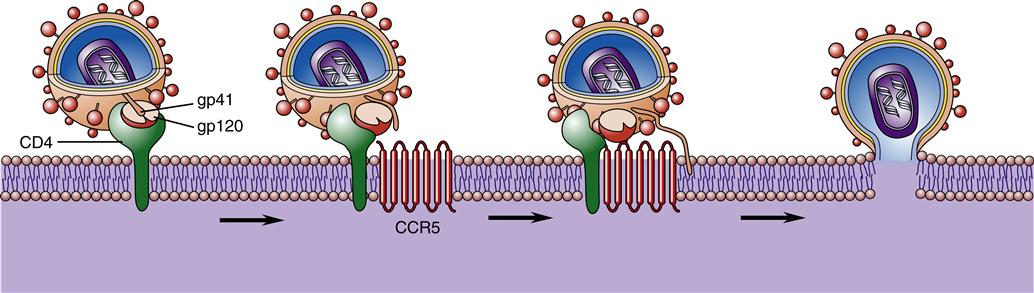
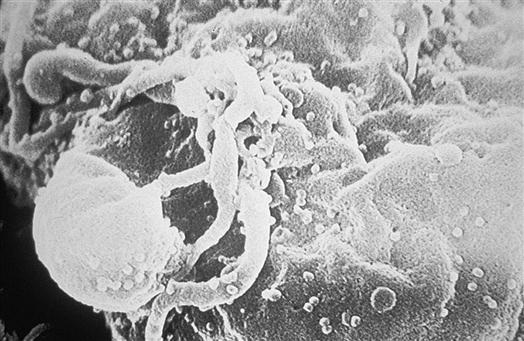
Once inside the body, HIV particles are attracted to cells with receptors on their surface called CD4. The HIV envelope protein gp120 specifically binds to the CD4 receptor. The specific CD4+ cells that are attracted to the virus change over time. The CD4 receptor is found on many types of cells, including T cells, microglial cells, monocyte-macrophages, follicular dendritic cells, immortalized B cells, retinal cells, Langerhans cells in the skin, bone marrow stem cells, cervical cells, bone marrow–derived circulating dendritic cells, and enterochromaffin cells in the colon, duodenum, and rectum. Of these cells, the CD4+ T helper/inducer cells and macrophages are most often implicated and involved in the process of infection. Figure 12-4 illustrates a group of HIV-infected CD4+ cells imaged by scanning electron micrography. Initially the virus is attracted to macrophages and the virus is called “M tropic.” Later the virus either becomes dual tropic and affects both macrophages and T cells, or becomes “T tropic” and affects primarily T cells. During heterosexual transmission of HIV, the virus is attracted to Langerhans cells in the mucosal membranes mediated through the CD4/CCR5 pathway.12 Later the virus becomes attracted to other cells in the body, primarily T cells, and is “T tropic.”
Usually T cells are infected before the onset of symptoms. CD4+ T cells are composed of two subsets: T helper-1 (TH1) and T helper-2 (TH2). The TH1 subset produces interferon-γ and interleukin-2 (IL-2). The TH2 subset produces IL-4, IL-6, and IL-10. Of these two subsets, the one that is markedly decreased in advanced disease is TH1.
However, CD4 alone is not sufficient for fusion of the virion and host cell. A number of important coreceptors on the target cells called chemokines are necessary for the virus to gain entry into cells.13 These important chemokine coreceptors must be present for the virion to fuse with the host cell. The chemokine called CCR5 must be present for the HIV particles to bind to the CD4+ cells in early infection during the M-tropic phase, and another chemokine receptor named CXCR4 must be present in later infection during the T-tropic phase.13 Since 1996 when the coreceptors were first discovered, a number of other coreceptors have been identified, including APJ, CCR2b, CCR3, CCR8, CCR9, CX3CR1, CXCR4, GPR1, GPR15, STRL33, US28, and V28. The function of most of these coreceptors is unknown. It is hypothesized that some of the coreceptors may be needed for various strains of HIV, for HIV infection in infants and children, or for infection of the brain and nervous system.
The gp120 portion of the virion envelope must combine with the first receptor, CD4, and then change shape by refolding. In the second shape, it combines with the second receptor, either CCR5 or CXCR4, to fuse with the cell. Once the HIV particle is bound to both the CD4 receptor and the chemokine receptor on the host cell, gp41 implants itself in the cell membrane (Figure 12-5). This sequence of events causes the viral particle and the cell to fuse. The core of the virus is then injected into the cytoplasm of the host cell and infection is produced. The gp120 portion of the virion envelope is “hyper-variable in sequence,” which allows for the flexibility of the protein loops that permits the virion to escape neutralizing antibodies.13
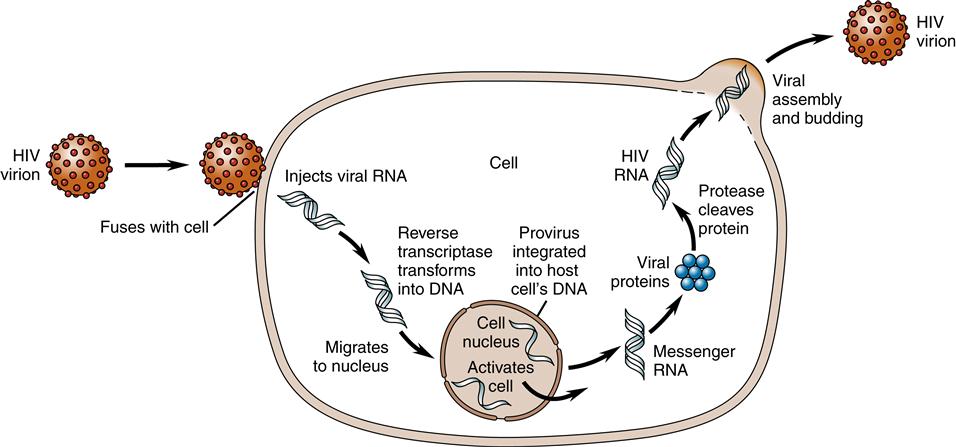
Once in the cytoplasm, a single-stranded DNA copy is made by reverse transcriptase from the viral RNA. Using the single-stranded DNA as a template, DNA polymerase copies it to make a second DNA strand and destroys the original RNA strands. The accuracy of DNA transcription is poor, with mutations occurring frequently. This tendency to mutate makes HIV highly resistant to antiviral medications.
Once formed, the new viral DNA, called viral provirus or preintegration complexes (PICs), migrates to the cell nucleus and is actively transported in the nuclear compartment.14 Inside the nucleus, integrase splices the viral DNA, or provirus, into the host cell’s DNA. Once in the host cell’s DNA, the viral DNA is replicated together with the host cell’s DNA during every cell division. Now the viral DNA is permanently part of the host cell’s DNA (Figure 12-6).
The process of building new virus particles begins within the host cell’s DNA. Segments at the end of the viral genome instruct the host cell to make RNA copies of the viral DNA. Some of the genes direct the host cell to manufacture viral envelope proteins (gene name: env) and enzymes (gene name: pol), whereas other RNA strands become future genetic material (gene name: gag). The HIV DNA then hijacks cellular protein pathways to produce the proteins needed for replication of HIV.14 In the nucleus of the cell, there is an interaction between the host cellular kinases and the HIV DNA that affects the HIV replication cycle.14–15 Sometimes the host cell produces kinases or factors that aid viral integration and sometimes there are kinases or factors that inhibit viral integration by recognizing and neutralizing infecting retroviral DNA.15 New studies are also demonstrating that not all proviral DNA is integrated into the cell’s DNA and yet the proviral DNA can still synthesize viral gene products and replicate within the cell assisted by the expression of tat and nef.15
To cause further infection, the viral RNA must be produced, leave the nucleus, and migrate to the cell surface. Rev proteins along with a human protein called CRM1 aid in the process of transporting the viral RNA proteins from the cell nucleus. A human RNA helicase enzyme, DDX3, helps to straighten HIV’s twisted strand of RNA before threading it through a small pore in the nucleus. The assembly of new virus particles, called virions, occurs at the cell membrane. Three proteins are produced and migrate to the cell periphery, attach to the cell membrane, and cause the viral material to bud out from the membrane. The protein-cutting enzyme protease separates the envelope proteins from enzymes and RNA genetic material and binds the viral core (Figure 12-7). Therefore, the completed virion has a host cell membrane from which the envelope proteins gp120 and gp41 protrude like spikes.
When CD4+ cells decline, the diversity of CD4+ cells is affected. With antiretroviral therapy, the naive T cells that can respond to new infections persist in low numbers despite an increase in memory T cells. Therefore, persons with HIV who are receiving antiretroviral therapy can respond to old but not new infections. This phase is indicative of deterioration in immune system function despite any temporary increase in CD4+ cell counts and decreased viral load from antiretroviral therapy.
Exposure to HIV-1 in epithelial cells (genital and gastrointestinal) and subsequent transmission and infection following exposure are incompletely understood. These processes involve multiple and complex interactions between HIV, cytokines, and CD4 cells. With HIV exposure, proinflammatory cytokines are produced by epithelial cells. Among the proinflammatory cytokines, tumor necrosis factor-α (TNF-α) is produced, which impairs the tight epithelial junctional barrier and allows migration of HIV and bacteria to move or translocate across the epithelium.15 Infection in the presence of inflammation is also influenced by the presence of CD4+ macrophages or Langerhans cells that lie directly under the epithelial cells. Macrophages may also release tumor necrosis factor or other cytokines stimulating other antigen-presenting cells, or T cells.16
Pathogenesis
Effect of HIV on Immune Cells at the Cellular Level
The hallmark of HIV infection is the decrease in the number of CD4+ T helper/inducer lymphocytes. T helper/inducer cells are necessary for appropriate immune responsiveness because they are the cells that mediate between the antigen-presenting cells, other immune cells such as B cells, and other T cells. During acute and chronic untreated HIV infection, the immune system is in a hyperactive state with high T-cell death, nonspecific T cell activation, polyclonal activation of B cells, and elevated levels of proinflammatory cytokines.17
Macrophages have CD4 receptors and act as both targets and reservoirs for HIV. As the infection progresses, they become more functionally impaired with defective phagocytosis and chemotaxis, abnormal antigen presentation, and abnormal cytokine production. They also contribute to the T-cell decline by increasing CD4+ cell death.
Humoral immune system dysfunction is also present, although the effect of HIV on antibody-producing B cells is more poorly understood. There are changes in B cell structure and function. B cell numbers usually remain normal but they are progressively dysfunctional with overproduction of nonessential antibodies, as well as failure to respond appropriately to normal immune system signals.18 Immunoglobulin G1 (IgG1) and IgG3 levels are usually elevated, causing hypergammaglobulinemia. IgM levels are elevated in early infection, whereas IgA levels are elevated in late infection. Despite these elevations, the responsiveness to bacterial cell wall (polysaccharide) antigens that require CD4+ cell activation of B cells is decreased. Immune complexes are increased, and B-cell differentiation and response to antigens are decreased. Autoantibodies, especially against erythrocytes, platelets, lymphocytes, neutrophils, nuclear proteins, myelin, and spermatozoa, occur either in association with disease processes (e.g., HIV-associated thrombocytopenia) or spontaneously. HIV antibodies are produced, but they are ineffective against the disease. Also B cells have an increased risk of cell death through apoptosis.16
The envelope glycoproteins (gp120 and gp41) on the surface of HIV virions are the reason for successful HIV infection. The immunogenic portions of the viral envelope glycoproteins are well disguised and variable—most likely because of the large amount of carbohydrate on the surface of gp120.13 Within the human body, high-carbohydrate substances look like “self” to the immune system. Therefore, the virus “hides” under the cover of the glycosylation. Another factor that allows HIV envelope proteins to escape the early antibodies is the way that gp120 and gp41 are bound together. Although the interface between gp120 and gp41 is an area that is highly immunogenic, the gp120 and gp41 molecules are noncovalently bonded together. Early antibodies cannot bind the assembled, functional envelope glycoprotein complex. Later, neutralizing antibodies are effective against the complex, but by that time the infection is well established.
Viral Production and Cell Death
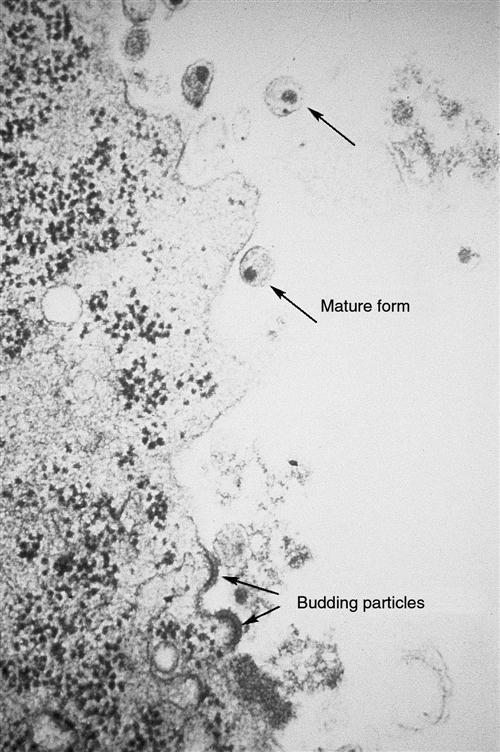
A key element in the success of HIV infection is that HIV replicates prolifically from the onset of infection. It generates so many virions that it overwhelms the body’s defenses. Because HIV is primarily a mucosal disease, the gastrointestinal (GI) tract is the major site of HIV replication.17 Within the first 3 to 6 weeks and continuing throughout the infection, HIV replication is high in the lamina propria CD4 T cells of the GI tract.17 HIV infection is characterized by a high level of virion turnover (HIV replication) and a high level of CD4+ cell turnover (host cell death). HIV-infected CD4+ cells undergo viral budding to generate and produce new virions (Figure 12-8). At least 10 billion HIV particles are produced and destroyed each day, with a plasma virus half-life of 6 hours and an acutely infected T-cell half-life of 1.1 days. Total T-cell numbers in acute HIV infection decline sharply, but with continuing infection blood T-cell numbers rebound slightly as a result of antiviral immune responses whereas GI T cell numbers remain low. In children infected with HIV, the virus is more aggressive and leads more rapidly to immune system dysfunction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree