INTRODUCTION
LEARNING OBJECTIVES
After studying this chapter you should understand:
The clinical applications and complications of hematopoietic stem cell transplantation.
The major types of hematopoietic stem cell transplantation.
The genetics of allogeneic hematopoietic stem cell transplantation.
The causes of graft rejection and graft failure.
The pathogenesis of graft-versus-host disease and the graft-versus-tumor effect.
The era of hematopoietic stem cell transplantation (HSCT) began in the 1950s, when pioneering studies by E. Donnall Thomas, James Till, and Ernest McCulloch demonstrated the ability of unfractionated bone marrow cells to “rescue” animals from hematopoietic failure induced by otherwise lethal doses of radiation. Such studies had profound implications and consequences for basic research, because they proved the existence of multipotent hematopoietic stem cells and provided a powerful new experimental tool for studying the immune system.
From very early days, it was evident that HSCT also had enormous therapeutic potential but was prone to cause serious and all too frequently fatal complications. HSCT remains as close to a high-wire act as exists in medicine, one in which patients receive potentially lethal doses of chemotherapy and/or radiation. However, as we will discuss, advances in stem cell biology, immunology, and pharmacology have allowed the development of more effective, less toxic HSCT strategies. As a result, HSCT is now being used to treat an increasing number of disorders and a broader spectrum of patients than ever before. This chapter serves as an overview of some of the salient features of this fascinating, rapidly evolving area of hematology.
CLINICAL APPLICATIONS OF HSCT
HSCT has proven to be effective in the following clinical settings:
Correction of genetic and acquired defects in hematopoietic stem cells (HSC). Until the dream of gene therapy for germline defects is realized, HSCT is often the only hope for those suffering from severe genetic disorders that affect the function of the HSC or its progeny. Replacing the defective HSCs of the patient with HSCs obtained from a normal donor can cure such diseases. HSCT has been used to treat many genetic diseases, including those affecting lymphocytes (eg, severe combined immunodeficiency, X-linked agammaglobulinemia), red cells (eg, severe thalassemia, sickle cell disease), and monocytes/macrophages (eg, Gaucher disease).
Support for high-dose cancer treatment. The dose-limiting toxicity of radiotherapy and many conventional chemotherapy agents is bone marrow failure due to ablation of HSCs. HSCT overcomes this limitation by providing the patient with healthy HSCs, which can completely reconstitute hematopoiesis and the immune system over a period of weeks to months.
Generation of graft-versus-tumor effect. When a recipient receives HSCs from another individual who is not an identical twin, the transplanted HSCs give rise to a “new” immune system that may recognize the patient’s tumor as non-self and mount an immune response against it. It is now clear that this graft-versus-tumor effect is a very important benefit of HSCT in certain types of cancer, particularly myeloid leukemias.
Generation of organ graft tolerance. HSCT has sometimes been used to alleviate solid-organ rejection. These types of procedures typically involve the transplantation of HSCs along with a solid organ, such as a kidney or a single lobe of the liver.
Salvage from genotoxic chemicals or radiation. To date, this indication has applied mainly to rare individuals exposed to nuclear power plant accidents (eg, Chernobyl). It is sobering to consider, however, that the United States government is currently expending large amounts of time and money planning for the consequences of a terrorist attack involving a so-called dirty bomb containing radioisotopes. Such an attack has the potential to cause severe bone marrow damage to hundreds or thousands of people, many of whom may need to undergo HSCT if they are to survive.
There are three types of HSCT. Autologous transplants are performed with the patient’s own HSCs. These have the advantage of avoiding immunologic complications but fail to generate the graft-versus-tumor effect and thus produce higher rates of relapse in those being treated for myeloid leukemias. Syngeneic transplants use HSCs from identical twins and have the same advantages and disadvantages as autologous transplants. Allogeneic transplants are done with stem cells obtained from a genetically distinct donor.
The most important determinants of the immunologic consequences of allogeneic HSCT are the class 1 and class 2 major histocompatibility complex (MHC) antigens, also known as human leukocyte antigens (HLA). You will recall that class 1 and class 2 MHC molecules present short peptides derived from intracellular and extracellular antigens, respectively. Although many genes in the MHC locus influence immunity, experience has shown that, for patients undergoing allogeneic HSCT, the most important are the HLA-A, HLA-B, and HLA-C class 1 antigens and the HLA-DR class 2 antigens. The roles of the class 2 antigens HLA-DQ and HLA-DP are less clear. The genes encoding these HLA antigens are closely linked on chromosome 6 and thus are inherited en bloc in what is referred to as a haplotype. An allogeneic HSCT in which the haplotypes of the donor and the recipient are identical is called a matched transplant. HSCT can be performed with one or two mismatched antigens, but the risks of the procedure are substantially higher than with an HLA-identical donor.
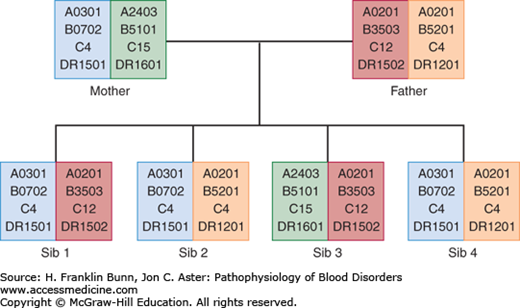
Another important variable in allogeneic transplantation is whether the HSCs are from a related family member or an unrelated donor. In addition to the MHC loci, poorly characterized “minor” histocompatibility complex loci have lesser but still significant immunologic effects. Allogeneic transplants from related donors are generally performed between siblings, who have a greater degree of identity at these minor loci than do unrelated donors. As shown in Figure 26-1, there is a 25% chance that any sibling will be fully matched at the MHC loci. In contrast, it is rare for a patient to have a full HLA match with a parent or a child. Of note, one or more of the minor histocompatibility complex antigens is located on the Y chromosome. As a result, the transplantation of HSCs from female donors into male recipients produces more graft-versus-tumor effect but also is more prone to result in significant graft-versus-host disease (GVHD) (described later).
FIGURE 26-1
Inheritance of HLA haplotypes. Note how different HLA-A, HLA-B, HLA-C, and HLA-DR alleles segregate together in this pedigree due to their close linkage on chromosome 6. Two of the children (sibs 2 and 4) are HLA identical, having inherited the same pair of haplotypes from their parents.
HSCs from several different sources are used clinically. Most allogeneic transplants from related donors are performed with whole, unfractionated bone marrow or with HSCs harvested from peripheral blood. The latter are typically obtained a few days after administration of a hematopoietic cytokine such as G-CSF (granulocyte colony stimulating factor (Chapter 2)). Allogeneic transplants from unrelated donors may be performed with bone marrow, HSCs harvested from the peripheral blood, or blood obtained from the placenta at birth (so-called umbilical cord blood). Cord blood has the advantage of being available as a byproduct of every child’s birth. There is a national initiative to establish public cord blood banks, with an eye toward future use in unrelated allogeneic HSCTs. The principle disadvantage of cord blood is that the number of HSCs in any sample is relatively small, a limitation that can be overcome by pooling of cord blood from two or more donors. Autologous transplantation is generally performed on cancer patients with peripheral blood HSCs obtained at 7-10 days after a dose of chemotherapy with GCSF stimulation. Recovery from the chemotherapy stimulates HSC proliferation and mobilization.