Fig. 2.1
Contraction-band artifact. Longitudinal (a) and cross-sectional (b) views of myocytes with contraction-band artifact. The contraction bands cause more and less eosinophilic regions that can mimic myocytolysis and/or coagulation necrosis
2.2 Acute Rejection
2.2.1 Acute Cell-Mediated Rejection (Figs. 2.2, 2.3, 2.4, and 2.5)
When cardiac transplantation was in its infancy, approximately 50 % of patients developed a hemodynamically significant episode of rejection during the first year after transplantation. Currently, at our institution, hemodynamically significant ACR occurs in less than 5 % of patients.
As with other solid organs, ACR in the transplanted heart is mediated by host cytotoxic and helper T-cells targeting graft antigens. One would therefore expect that T-lymphocytes would be the most numerous cells observed in the biopsy during an episode of rejection. In fact, our studies have shown that actually macrophages predominate. Usually, more than 50 % of the infiltrating cells are macrophages [2]. This finding turns out to be useful if IHC studies are performed to assist in the diagnosis of rejection versus QL. In ACR, most infiltrating cells will be macrophages with fewer T-lymphocytes and rare B-lymphocytes. In QL, most infiltrating cells will be B- or T-lymphocytes (depending on whether the top [B-cells] or bottom [T-cells] of the QL is biopsied, with only a relatively small proportion of macrophages. My personal practice is to order CD3, CD20, and CD68 on all biopsies suspected to have more than just mild rejection (grade 2R or 3R). C4d and CD31 (or 34) are also part of the package, as these immunostains are important in the evaluation of AMR to be discussed in a separate section.
In ACR, the first change noted is a perivascular infiltrate of mononuclear cells. These cells then spread out away from small blood vessels to involve interstitial tissue. As the ACR becomes more severe, myocyte injury becomes evident. Myocyte injury takes the form of coagulation necrosis only in very severe rejection. The more typical finding is that of “myocytolysis.” Affected myocytes will lose their sarcoplasmic organelles and the sarcoplasm appears empty. Nuclei enlarge and nucleoli become prominent. Atrophy of myocytes is also observed. These injured myocytes are not necessarily irreversibly injured and they may recover. Interestingly, after injury, the affected fibers demonstrate a fetal phenotype. By IHC, they express vimentin and smooth muscle actin, proteins that are not expressed in normal adult cardiac myocytes.
Probably all transplanted hearts at some time demonstrate some rejection of some degree. The pathologist must not only identify ACR but also grade the findings. The pioneering work in this regard was done by Dr. Margaret Billingham and colleagues [3] at Stanford, who devised the first grading system. There have been several grading systems since, but remarkably, the current system in use to diagnose ACR is very similar to Dr. Billingham’s first system.
Table 2.1 shows the criteria of grading for the current and most recent previous grading systems [4, 5]. The template we use at UCLA for reporting biopsy findings shows the criteria for each grade in both systems (See Appendix). We report both primarily for research purposes. The grade of rejection is based on the pattern of infiltrating cells and whether or not myocyte injury is present. This sounds easy, but artifacts in the tissue and lesions other than rejection can confound the interpretation.
Grade 0R (no ACR) is diagnosed when there is no inflammatory infiltrate and no myocyte injury. Rare interstitial or perivascular lymphocytes may be present.
Grade 1R (mild ACR) demonstrates focal perivascular infiltrates alone (previous 1A) or with some interstitial infiltrate as well (previous 1B) with no myocyte injury. A single focus of prominent cell infiltration that may be associated with myocyte injury is also considered grade 1R. This particular lesion was grade 2 in prior grading schemes. However, our studies, and those of others, have shown that these lesions are not rejection, but QLs [6]. This topic is still debated and some centers still regard these lesions as representing rejection.
Grade 2R (moderate ACR), former grade 3A, is defined by two or more foci of cellular infiltrates with myocyte injury. The important distinguishing feature of grade 2R ACR is multifocal myocyte injury that is not present in grade 1R (prior 1B) ACR.
Grade 3R (severe ACR) incorporates prior grades 3B and 4, and is characterized by diffuse inflammatory cell infiltrates that often contain eosinophils and neutrophils as well as lymphocytes and macrophages. Interstitial edema, hemorrhage, and vasculitis may be present, but in our experience, these findings are rare and more often observed in severe AMR. The difference between 2R and 3R is only the intensity and diffuseness of the process. The distinction is not critical, as in most transplant centers, both lesions are treated with augmentation of immunosuppression whether or not the patient is symptomatic or demonstrating graft dysfunction.

Fig. 2.2
Grade 1R ACR. (a) Prior grade 1A consists of a perivascular infiltrate of mononuclear cells. (b) Prior grade 1B shows mild interstitial as well as perivascular infiltration

Fig. 2.3
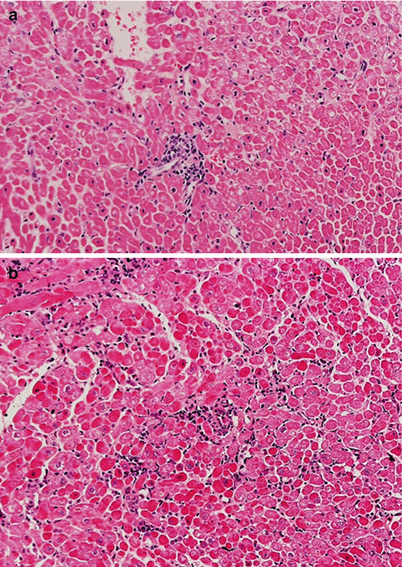
Grade 2R ACR (prior grade 3A). (a, b) Multifocal mononuclear cell infiltrates with myocyte injury

Fig. 2.4
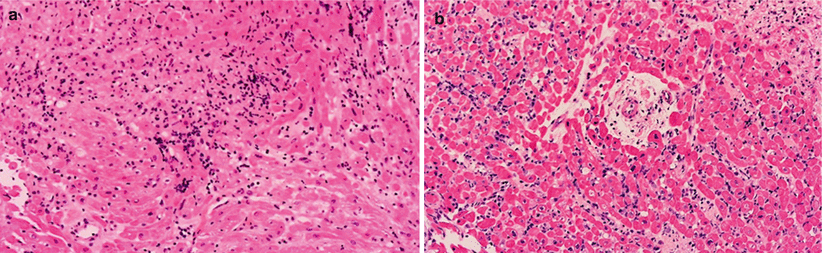
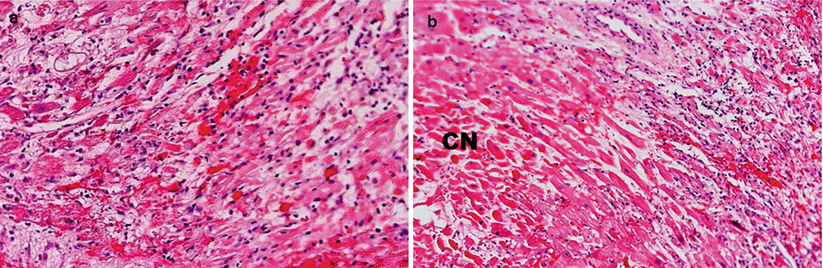
Grade 3R ACR. (a, b) Prior grade 3B with diffuse interstitial infiltrates with myocyte injury. (b) Prior grade 4. Note diffuse infiltrates, interstitial hemorrhage, and extensive myocyte injury. Areas with coagulation necrosis (CN)

Fig. 2.5
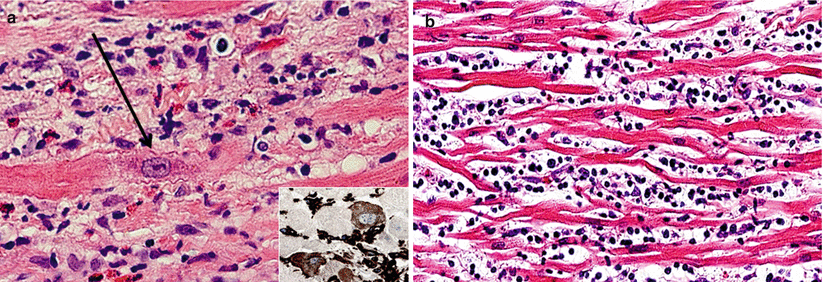
Myocyte injury: examples of myocyte changes associated with injury. (a) Clearing of sarcoplasm and enlarged nucleus with prominent nucleolus (arrow). (b) Marked atrophy. Inset, Vimentin immunoperoxidase stain
Grade 0 R | No evidence of cell-mediated rejection |
|---|---|
Grade 1 R, mild | Interstitial and/or perivascular infiltrate with up to 1 focus of myocyte injury |
Previous grade 1A: Perivascular infiltrates without myocyte injury | |
Previous grade 1B: Perivascular and sparse interstitial infiltrates without myocyte injury | |
Grade 2 R, moderate | Two or more foci of infiltrate with associated myocyte damage |
Previous grade 3A: Multifocal prominent infiltrates and/or myocyte injury | |
Grade 3 R, severe | Diffuse infiltrate with multifocal myocyte damage ± edema ± hemorrhage ± vasculitis |
Previous grade 3B: Diffuse infiltrates with myocyte injury | |
Previous grade 4: Diffuse polymorphous infiltrate with myocyte injury ± hemorrhage ± edema ± vasculitis |
2.2.2 Antibody-Mediated Rejection (Figs. 2.6, 2.7, 2.8, and 2.9)
AMR in cardiac transplantation was unrecognized for many years, and underrecognized for many more. Dr. Elizabeth Hammond and colleagues [7] at the University of Utah deserves credit for describing the clinical and pathological findings of AMR and emphasizing the importance of AMR. Before this work, the very existence of AMR had been questioned by prominent cardiac transplant physicians, including pathologists.
In AMR, graft injury results from antibodies, usually directed against antigens on endothelial cells that may initiate complement activation. With the decreasing incidence of ACR, AMR is now more common, affecting 10–20 % of patients, depending on the transplantation population. AMR is more common in “sensitized” patients, such as multiparous females, patients with prior blood transfusions, and those who have had implanted ventricular-assist devices. Typically, AMR is seen soon after transplantation in sensitized individuals, but can occur months or years later, especially in patients with no prior sensitization. AMR can cause hemodynamic dysfunction, accelerated allograft vasculopathy, graft loss, and death.
The histological hallmark of AMR is dilation of capillaries by intraluminal cells that has been described as “endothelial swelling.” Electron microscopic and IHC studies have shown that the majority of these cells are actually macrophages and not endothelial cells [8]. In addition to clarifying the nature of the process, this recognition is of practical importance; demonstrating intravascular macrophages by IHC staining is very helpful in making the diagnosis of AMR. CD31 or CD34 staining highlights capillary dilation and confirms the intravascular location of these cells, as opposed to the predominantly perivascular location of infiltrating cells in ACR.
C4d and/or C3d staining on capillaries that demonstrates complement deposition, can further confirm the diagnosis. Because the histological findings of AMR can be very subtle, sometimes the only evidence of AMR is by IF or IHC studies; therefore, it is recommended that these studies be done routinely early after transplantation or if the patient has unexplained cardiac dysfunction (Tables 2.2 and 2.3) [9].
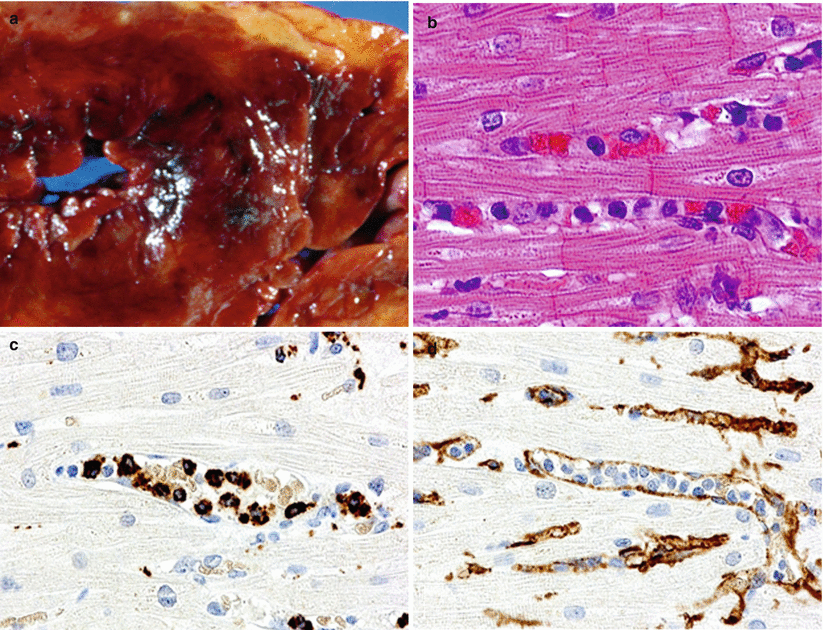
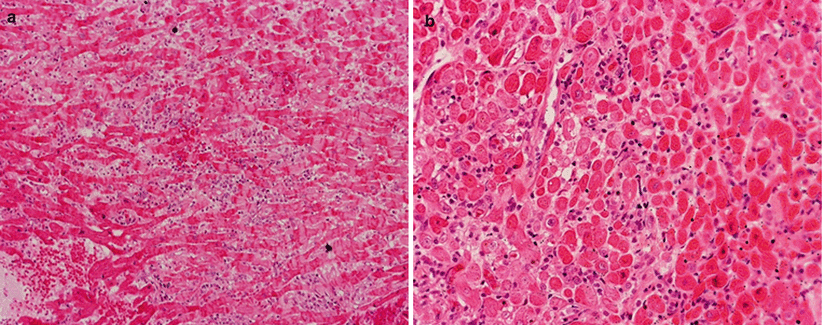
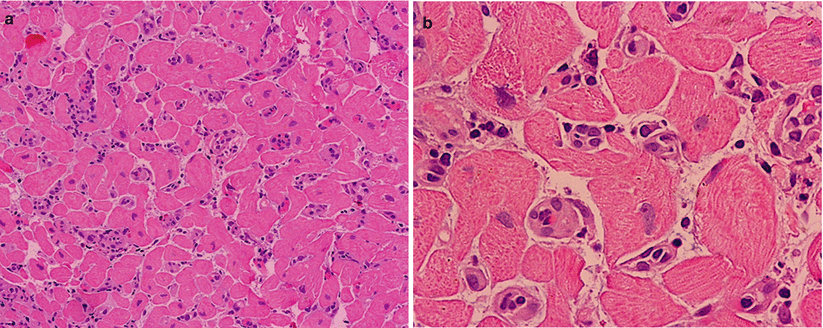
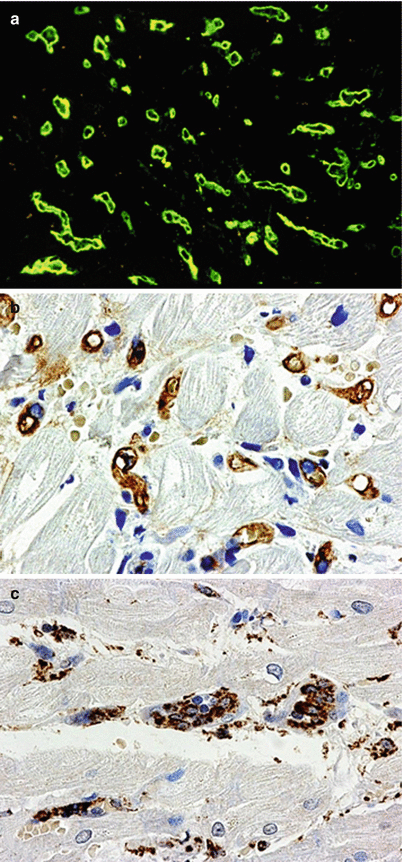

Fig. 2.6
AMR. (a) Gross photograph of a patient who died in cardiogenic shock 7 days after transplantation. Note hemorrhagic necrosis of the myocardium. (b) Note intravascular mononuclear cells. (c) CD68 immunostain confirms the presence of intravascular macrophages. (d) CD31 stain confirms the intracapillary location of macrophages

Fig. 2.7
AMR. (a, b) Low-power views show a hint of AMR because the interstitium is more cellular than normal, but they do not show clusters of lymphoid cells

Fig. 2.8
AMR. (a) Low-power view shows interstitial mononuclear cells. (b) High magnification shows that the mononuclear cells are within, not around, the capillaries

Fig. 2.9




AMR IH. Examples of IHC findings in AMR. (a) Positive IF staining for C4d in capillaries. (b) Positive immunoperoxidase staining for C4d in capillaries. (c) Positive IHC staining for macrophages (CD68) within capillaries
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


