Fig. 7.1
Comparison of halo nevus (a) and superficial spreading melanoma with focal regression (b). Low magnification; exophytic, symmetrical melanocytic lesion with a dense, regularly distributed, lichenoid infiltrate that obscures the dermal–epidermal junction and infiltrates among dermal melanocytic nests and nevus cells. There is focal effacement of rete ridges. Scattered melanophages are located in papillary and superficial reticular dermis (a). Superficial spreading melanoma, low magnification, can mimic of halo dysplastic nevus with asymmetrical feature and irregular elongation of rete ridges and variable junctional melanocytic nests extended to the periphery of the lesion with bridging pattern, focally prominent fibrosis of subjacent papillary dermis in the center of the lesion suggestive of focal regression. The lichenoid infiltrate is unevenly distributed along the lower portion of the tumor without infiltrate into the melanocytic nests (b). Halo nevus, compound type: predominantly dermal melanocytic nests admix with lymphocytes throughout the lesion; minimal pagetoid upward migration is observed in the center of lesion. Scattered small lymphocytes are present in dermal melanocytic nests with mild cytological atypia. Mitotic figures are not identified in this halo nevus (c). Contiguous single cell proliferation and irregular junctional nests in in situ melanoma. Fibrosis, increase dermal vasculature, and scatter lymphocytes with reduced dermal invasive component are suggestive of melanoma with focal regression. There may be marked cytologic atypia with pagetoid upward migration of individual atypical melanocytes (d, e). Nodal metastatic melanoma demonstrating the same tumor phenotypes of epithelioid cells with markedly atypia and increased mitotic figures in the same patient (f)
Other differential diagnosis of halo nevus includes:
Dysplastic nevus with halo phenomenon (halo dysplastic nevus): characteristic features include remnants of a compound dysplastic melanocytic nevus with some degree of architectural disorder and cytological atypia. There may be a superposition of the features of host response typical of dysplastic nevus (lamellar and concentric fibroplasia, and concentric fibroplasia, and melanophages) (Fig. 7.2). Clinically, there may be well-defined, hypopigmented or depigmented halo.
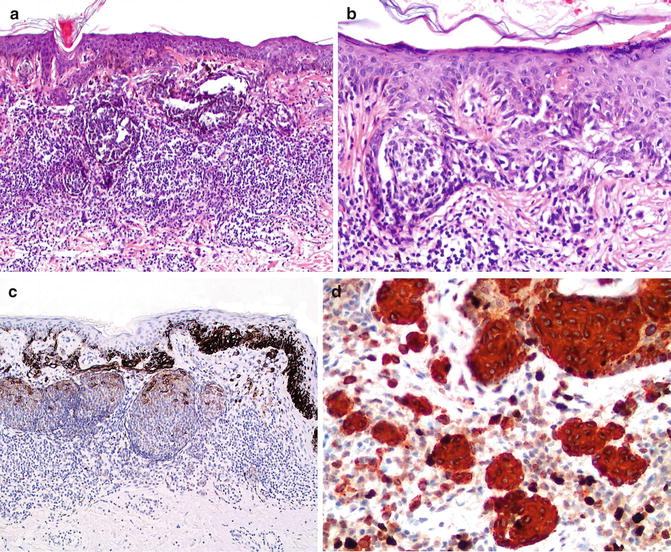
Fig. 7.2
Halo dysplastic nevus with a dense lichenoid infiltrate that obscures the dermal–epidermal junction (a). Features of lentiginous pattern and bridging junctional nests with mild cytological atypia and lamella fibrosis at subjacent papillary dermis are observed (b). HMB-45 is strongly positive in junctional melanocytic nests and intraepidermal single melanocytes, and much weaker in dermal melanocytic nests and dermal nevus cells (pattern of maturation with HMB-45 in benign nevus) (c). High-power; Ki67/MART-1 double immunostudy highlights minimal dermal proliferation in dermal melanocytic components (d)
Spitz nevus with halo phenomenon preserves the structure of dome-shaped, well circumscribed, nested, symmetrical, with epidermal hyperplasia, compact orthokeratosis, eosinophilic globules (Kamino bodies), and clefting between junctional nests and adjacent epidermis. Nevus cells are spindled or epithelioid, with decreased size with depth in the dermis. Mitotic figures are usually superficial. There may be either diffuse labeling or loss of labeling with HMB45 as opposed to patchy positivity in the dermal component of melanoma. Molecular study may be helpful, since Spitz nevi only exceptionally may show homozygous deletion of 9p21.
Congenital melanocytic nevus with halo phenomenon is very rare and has infrequently been associated with melanoma [18–20]. As with all congenital melanocytic nevi with unusual features, long-term follow-up is very important.
Meyerson nevus [21, 22] is a nevus with eczematous halo reaction. This nevus is different, clinically and histologically, from halo nevi. Clinically, there is erythema (eczematous halo) surrounding the nevus without area of depigmentation. Histologic findings include epidermal acanthosis with eosinophilic spongiosis and occasional intraepidermal vesicle formation. The dermis contains a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils surrounding the nevus. There may be occasional cytologic atypia of melanocytes, e.g., hyperchromatic, irregular nuclei.
Halo reaction or band-like lichenoid infiltrate has been described in other non-melanocytic lesions, such as seborrheic keratosis, lichen planus, benign lichenoid keratosis, keratoacanthoma, keloid, insect bites, dermatofibroma, basal cell carcinoma, and squamous cell carcinoma [4, 23, 24]. In all these lesions, examination of histologic features is usually sufficient to establish the correct diagnosis. However, immunohistochemistry may be needed in cases in which the infiltrate completely obscures the dermal–epidermal junction and it is unclear if there is a proliferation of melanocytes (see below).
Immunohistochemistry
In general, anti-MART-1 and HMB-45 will highlight intraepidermal and dermal melanocytes. However, it has been described that both antibodies may label keratinocytes, presumable due to transfer of melanosome antigens to keratinocytes [25]. In such cases, nuclear markers such as microphthalmia transcription factor (MiTF) or SOX-10 may be more specific. Anti-S100 antibody labels both melanocytes and Langerhans cells; the latter may be interpreted to be pagetoid melanocytes (the presence of dendritic cytoplasmic processes would be unusual in pagetoid melanocytes). Loss of HMB-45 labeling with depth in the dermis suggests a pattern of maturation of the nevus cells, thus supportive of a diagnosis of nevus. The proliferative marker Ki67 (MIB-1) can be slightly increased in intradermal/junctional component of the lesion but should be negative in deep-dermal located melanocytic nests in benign nevi. The use of a double immunoreaction (anti-Ki67 and anti-MART-1) will help distinguish proliferating melanocytes from intermixed lymphocytes (Fig. 7.3).
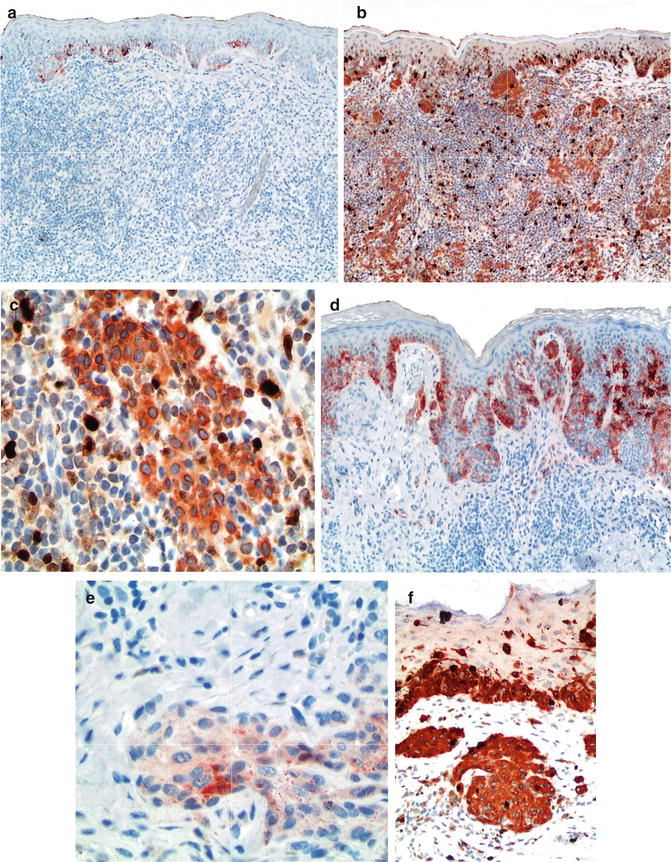

Fig. 7.3




Use of HMB-45 and Ki67/MART-1 double stain to differentiate between benign melanocytic nevi with halo reaction and melanoma with regression. HMB-45 is strongly expressed in intraepidermal nests but lost in the dermal component of halo nevus, thus consistent with maturation (a). In contrast, it shows patchy and irregular labeling pattern in dermal nests of melanoma (b, c), Ki67/MART-1 double stain; melanocytic component in both intraepidermal and dermal location are detected by cytoplasmic stain pattern of MART-1 without increased proliferative index in lesional melanocytes. Nuclear pattern of expression by Ki67 is detected with increased expression in basal keratinocytes and lymphocytic infiltrate but not melanocytic cells (d, e), in contrast to increased proliferative index in dermal melanocytic cells of melanoma (f)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


