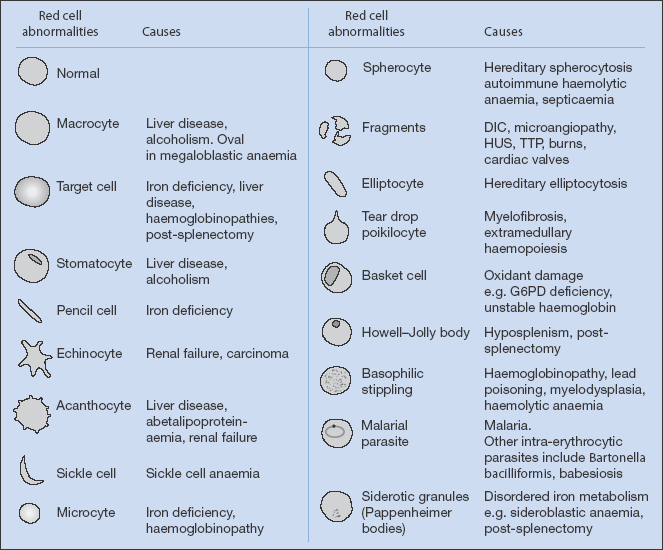
Peripheral Blood Film Features (Fig. 20.1)
- reticulocytes (active marrow) – haemolysis or chronic blood loss
- anisocytes (variation in red cell size) or poikilocytes (variation in red cell shape) – iron deficiency
- target cells (‘Mexican hat’ cells) – thalassaemia
- rouleau formation (clumping together of red cells) – raised ESR (check for myeloma)
- burr cells (echinocytes with irregular ‘crinkled’ red cell membrane) – renal failure, carcinoma
- hypersegmented polymorphs – vitamin B12 or folic acid deficiency
- Howell–Jolly bodies (remnants of nuclear material) – splenectomy (or non-functioning spleen)
- blast cells (immature cells) – acute leukaemia
- eosinophilia – parasitic infection, allergy, occasionally systemic vasculitis or Hodgkin’s disease
Figure 20.1 Morphology of red cells. (From Mehta and Hoffbrand (2009) Haematology at a Glance, 3rd edition Wiley-Blackwell, Oxford.)
Reticulocytes
Normal range is 10–100 × 109/l. Reticulocytes are premature red cells in which traces of nucleoprotein remain as fine, reticular strands. They are larger than mature red cells and, if increased, may cause macrocytosis. An increase (reticulocytosis) suggests marrow hyperactivity because of:
- loss or destruction of red cells, e.g. bleeding
- a response to treatment of anaemia, e.g. of pernicious anaemia with vitamin B12
- haemolysis.
Normocytic Anaemia
Mean corpuscular volume (MCV) is in the normal range. Usually anaemia is secondary to chronic disease. It is usually insidious, not progressive and fairly mild (> 9 g/dl) except in chronic kidney disease. It may become slightly hypochromic and/or microcytic. The white cell count and platelets are normal. The serum transferrin is normal or low but, unlike iron deficiency, the serum ferritin is normal or high (with increased iron stores in the bone marrow). A marrow examination may show malignant disease (leukaemia, mycloma, metastasis) or myelofibrosis.
Anaemia of chronic diseases occurs in:
- chronic kidney disease (p. 159) – check serum creatinine and estimated glomerular filtration rate (eGFR, p. 160)
- chronic liver disease – check liver function tests, γ-glutamyl transferase, prothrombin time
- auto-immune disease (e.g. rheumatoid arthritis, systemic lupus erythematosus (SLE)) – check ESR, C-reactive protein and autoantibodies: rheumatoid factor, anti-nuclear antibodies and if positive specific tests for antibodies against nuclear antigens (p. 279), anti-neutrophil cytoplasm antibodies (ANCA; present in systemic vasculitis)
- chronic infection – abscesses, tuberculosis, bacterial endocarditis
- cancer.
NB The anaemia of chronic kidney disease can be effectively reversed by treatment with recombinant human erythropoietin (p. 162). Erythropoietin can also reverse anaemia associated with cancer, although concerns have been raised that erythropoietin may contribute to tumour progression.
Microcytic Anaemia
MCV is low, e.g. < 80 fl. The serum iron is either low (iron deficiency) or normal (haemoglobinopathies, usually thalassaemia minor, p. 329). The mean corpuscular haemoglobin (MCH) is usually low (hypochromic), i.e. < 25 pg.
Iron deficiency is caused by poor intake, poor absorption, poor iron use by the marrow or increased blood loss (menstrually or from the gut). Check with serum iron (very low) and transferrin, which tends to be high. If in doubt check the serum ferritin (low) and demonstrate low iron stores in the marrow.
Macrocytic Anaemia
MCV is raised, often > 100 fl.
- vitamin B12 deficiency (usually pernicious anaemia) – check serum B12
- folic acid deficiency – check red cell folate
- hypothyroidism – check thyroid function tests
- liver disease (usually excess alcohol) – check liver function, including γ-glutamyl transferase
Pernicious anaemia is now usually diagnosed by finding low serum vitamin B12 with parietal cell and intrinsic factor antibodies, rather than with the Schilling test (B12 absorption before and after intrinsic factor). Check the haemoglobin, and reticulocyte response to therapy. If in doubt, marrow examination may provide a definitive diagnosis (megaloblastic).
Anaemia Secondary to Chronic Disease
Anaemia about 10 g/dl, usually normocytic, is associated with chronic infection, malignant disease, chronic kidney disease and chronic inflammation. The serum iron is characteristically reduced, and so is the transferrin (iron-binding capacity), unlike the findings in iron-deficiency anaemia. The marrow iron stores are increased, but the iron is not incorporated fully into red cell precursors.
Pancytopenia
This is a rare combination of anaemia, leucopenia and thrombocytopenia. It is caused by either:
- reduced production of cells, caused by:
 bone marrow infiltration (leukaemia, myeloma, carcinoma, myelofibrosis)
bone marrow infiltration (leukaemia, myeloma, carcinoma, myelofibrosis) bone marrow aplasia: idiopathic or drug-induced (e.g. NSAIDs, chloramphenicol, chemotherapy for malignancy); severe vitamin B12 or folate deficiency
bone marrow aplasia: idiopathic or drug-induced (e.g. NSAIDs, chloramphenicol, chemotherapy for malignancy); severe vitamin B12 or folate deficiency- increased destruction of cells, caused by hypersplenism; or autoimmune disease (e.g. SLE).
Bone marrow examination is the most important investigation in distinguishing these causes.
Marrow Suppression
Secondary bone marrow failure may affect one or all of the formed elements of the blood – red cells, white cells or platelets. It may be idiopathic or secondary to infiltration, drugs, (gold, penicillamine, chloramphenicol, carbimazole), radiation, leukaemias, infections or other disorders such as uraemia, hypothyroidism and chronic disease.
Erythrocyte Sedimentation Rate (ESR)
ESR measures the rate of sedimentation (in millimetres per hour) of red cells in a column of anticoagulated blood. Rapid sedimentation (increased ESR) suggests increased levels of immunoglobulins or acute phase proteins, which cause the red cells to stick together. A raised ESR is therefore a non-specific indicator of inflammation or infection. The ESR is usually very high in myeloma.
A very high ESR (> 100 mm/h) suggests:
- multiple myeloma
- SLE or vasculitis
- temporal arteritis
- polymyalgia rheumatica
- rarely, carcinoma or chronic infection, including tuberculosis.
Anaemia
There are three major types of anaemia, classified by cause: deficiency, haemolysis and marrow disorders. The symptoms are tiredness, physical fatigue and dyspnoea, with angina, heart failure and confusion in older people.
Anaemia can be caused by a deficiency in:
- iron
- vitamin B12
- folic acid.
Iron-Deficiency Anaemia
Diagnosis
The cause of the iron deficiency must be identified and corrected. In premenopausal women, excess menstrual loss is often the cause, although this should not be accepted uncritically because other important causes may be present as well. Slow gastrointestinal loss is a common cause, with peptic ulceration, gastric carcinoma and carcinoma of the descending colon most common. Carcinoma of the ascending colon or caecum frequently produces no symptoms and its presence must be considered in all cases of iron-deficiency anaemia. In the elderly, dietary deficiencies remain an important cause, and remember that hypothyroidism can present as iron-deficient anaemia.
Examination
This includes assessment of pallor (very imprecise), glossitis, angular stomatitis, koilonychia and rectal examination. Investigate the gastrointestinal tract if no other cause is identified. Early colonoscopy, especially in the asymptomatic patient, can detect carcinoma of the large bowel at a curable stage.
Laboratory Investigation (Table 20.1)
The peripheral blood count shows hypochromia (MCH < 27 pg) and microcytosis (MCV < 80 fl), possibly with poikilocytosis (variation in shape) and anisocytosis (variation in size). The serum iron is low and the transferrin raised, with a low saturation. The serum iron is also low in anaemia secondary to chronic disease, but normal in haemoglobinopathies and, usually, thalassaemia minor (p. 329). Serum ferritin reflects the state of the iron stores and is therefore low. There is a reduction in stainable iron in the marrow. The bone marrow shows adequate iron in macrophages but reduced amounts in developing erythroblasts. Thalassaemia (p. 329) also causes hypochromic, microcytic anaemia.
Table 20.1 Haematological reference values in anaemia. A significant difference between one Hb reading and the next is 1 g/dl. If you are attempting to analyse anaemia and are looking at a Coulter-style full blood count, first check the MCV. See if the cells are normal (normocytic), small (microcytic) or large (macrocytic)
| Hb (male) | 12.5–16.5 × 109/l |
| Hb (female) | 11.5–15.5 × 109/l |
| An automated cell counter (e.g. Coulter counter) typically gives the following readings: | |
| Haematocrit (PCV; male) | 0.42–0.53 |
| Haematocrit (PCV; female) | 0.39–0.45 |
| Red cell count (male) | 4.5–6.5 × 1012/l |
| Red cell count (female) | 3.9–5.6 × 10112/l |
| MCV | 80–96 fl* |
| RDW | 11.1–13.7†† |
| MCH | 27–31 pg‡ |
| MCHC | 32–36 g/dl§ |
| Transferrin (iron-binding plasma protein) | 2–3 g/l |
| Raised in iron deficiency (and pregnancy) | |
| Reduced in anaemia of chronic disease, acute inflammation and protein loss | |
| Ferritin | Correlates with tissue iron stores (iron is stored in the tissues in two forms, ferritin and haemosiderin). Only low in iron-deficiency states |
| Hb, haemoglobin; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; PCV, packed cell volume; RDW, red cell distribution width. | |
| *MCV is haematocrit/red cell count. | |
| †This is an automated measure of anisocytosis: the variability of red cell size. | |
| ‡MCH is Hb/red cell count. | |
| §MCHC is Hb/haematocrit. MCH and MCHC are of limited use in the differential diagnosis of anaemia. | |
Management
In the absence of active bleeding, ferrous sulphate 200 mg b.d. before food is usually all that is required. The reticulocyte count rises first and then the haemoglobin (at about 1 g/week), but iron should be continued for another 3 months to replenish the stores.
NB Hypochromic anaemia, unresponsive to oral iron therapy, occurs in:
- incorrect diagnosis or mixed deficiency
- continued bleeding (reticulocytosis persists), e.g. microscopic from tumour of the bowel
- patients who do not take their tablets
- rheumatoid arthritis (p. 270)
- malabsorption (p. 136)
- thalassaemia (p. 329)
- myelodysplastic syndrome (p. 330) – refractory anaemia (if ringed sideroblasts present in marrow, sideroblastic anaemia – p. 330).
Hazards of Blood Transfusion
- Transfusion reaction – minimise risk by cross-matching patient’s serum with donor blood. If clinical manifestations of a transfusion reaction occur (fever, backache, hypotension and haemoglobinuria), stop the transfusion immediately and initiate supportive treatment to alleviate shock.
- Transmission of infection – blood is screened for hepatitis B and C and human immunodeficiency virus (HIV).
- Circulatory overload – give furosemide with transfusion in patients at risk of heart failure.
- Coagulation defects and electrolyte abnormalities – particularly hyperkalaemia (red cell breakdown releases potassium) where large volumes are transfused.
Vitamin B12 Deficiency (Usually Pernicious Anaemia)
Vitamin B12 is present in liver, and small amounts also in milk and dairy products, and requires intrinsic factor for absorption. The most common cause of vitamin B12 deficiency in the UK is lack of intrinsic factor as a result of parietal cell and intrinsic factor antibodies. It is associated with other organ-specific autoimmune disorders. Achlorhydria is invariably present. Rare causes of B12 deficiency include gastrectomy, intestinal blind loops (in which bacteria multiply using up B12), a vegan diet, Crohn’s disease involving the absorbing surface in the terminal ileum, other causes of malabsorption and Diphyllobothrium latum Finnish tapeworm that consumes B12. Stores of B12 last 3–4 years.
Clinical Features
Pernicious anaemia occurs in the middle-aged and elderly and is more common in women. Exhaustion and lethargy are the most common presenting complaints, although pallor may be noticed incidentally, or the blood picture noticed in the laboratory.
In chronic, severe B12 deficiency, which is uncommon, the skin has a pale lemon tint, the hair is snow white and the sclera may be slightly jaundiced as a result of mild haemolysis. The tongue may be tender, smooth and red because of atrophy of the mucosa. Peripheral neuropathy may be the presenting feature with pain, soreness or numbness of the feet on walking. Later, features of subacute combined degeneration of the cord may develop. Cardiac failure is common if the anaemia is marked. The spleen is sometimes palpable. There is an increased incidence of gastric carcinoma.
Diagnosis
The haemoglobin may be very low, i.e. 3–4 g or less. The blood film shows macrocytes usually with anisocytosis and poikilocytosis, and the MCV is usually > 100 fl. The total white blood cell (WBC) count may fall because of reduced numbers of both lymphocytes and neutrophils (Table 20.2). Some neutrophils may show hypersegmentation of the nuclei (> 5 lobes). There may also be a moderate fall in the platelet count. Reticulocytes are generally not increased until treatment is started.
Table 20.2 White cells. Normal White Cell Count: 4–10×109/l
| Neutrophils – normal range: 2.0–7.5 × 109/l (40–75% of total white cells) |
| Causes of neutrophilia (raised neutrophil count) |
| • Acute bacterial infections |
| • Inflammation, e.g. arteritis |
| • Acute tissue necrosis, e.g. myocardial infarction, large pressure sores, burns |
| • Acute haemorrhages |
| • Leukaemias |
| Causes of neutropenia (low neutrophil count) |
| • Viral infections, e.g. glandular fever, measles, acquired immunodeficiency syndrome (AIDS) |
| • Drug reactions, e.g. carbimazole, chemotherapy |
| • Blood diseases, e.g. leukaemias, pernicious anaemia, aplastic anaemia |
| Lymphocytes – normal adult range: 1.5–4.0 × 109/l (20–45% of total) |
| There are two main subpopulations of T lymphocytes, which bear different surface markers, or cluster of differentiation (CD) antigens. CD8 cells are ‘cytotoxic’ – their main function is to recognise and kill cells expressing foreign (usually viral) proteins. CD4 cells are ‘helper’ cells – they help B lymphocytes to differentiate into plasma cells and produce antibodies. The normal ratio of CD4 : CD8 cells is 2 : 1. |
| Causes of lymphocytosis (raised lymphocyte count) |
| • Acute viral infections, e.g. glandular fever, chickenpox, rubella, mumps |
| • Lymphatic leukaemia |
| • Vasculitis and drug hypersensitivity |
| Causes of lymphopenia (low lymphocyte count) |
| • AIDS – a severely depressed CD4 count predicts the onset of opportunistic infections |
| • Ionising radiation (treatment for malignancy or accidental) |
| • Chemotherapy for malignancy |
| • Steroid therapy or Cushing syndrome |
| Eosinophils – normal range: 0.04–0.4 × 109/l |
| Causes of eosinophilia (raised eosinophil count) |
| • Allergies, e.g. bronchial asthma, urticaria, hay fever, drug reaction |
| • Parasitic infestation of gut or other tissues (muscles, subcutaneous tissues, liver, urinary tract) |
| • Systemic vasculitis (see polyarteritis nodosa, p. 286; Churg–Strauss syndrome, p. 289); Hodgkin’s disease, p. 332 |
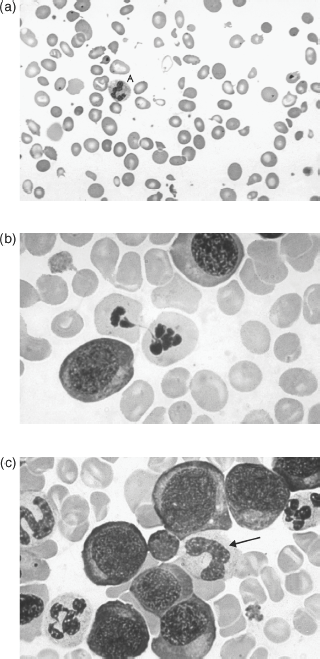
The marrow is hypercellular, with giant metamyelocytes and megaloblasts present – evidence that anaemia is in part caused by suppression of cell release. Megaloblasts (Fig. 20.2) are found only rarely in the peripheral blood. They are characterised by a large and inactive nucleus (maturation arrest) in a relatively hypermature, and even haemoglobinised, cytoplasm. They are not present in normal marrow and their presence denotes vitamin B12 or folate deficiency, which may be secondary to antifolate or phenytoin therapy. If sufficiently severe, vitamin B12 and folate deficiencies produce depression of all the marrow elements, including neutrophils and platelets. There is usually some haemolysis with a raised unconjugated serum bilirubin. The haptoglobins are reduced. Urobilinogen is present in the urine as a result of reduced red cell survival and ineffective erythropoiesis. Antibodies to parietal cells are present in > 90% of patients and to intrinsic factor in approximately 55%. Not all individuals who have parietal cell antibodies have pernicious anaemia.
Figure 20.2 (a) Peripheral blood in megaloblastic anaemia, showing a hypersegmented neutrophil (A), oval macrocytes and poikilocytosis (variation in red cell shape). (b) Bone marrow in megaloblastic anaemia showing megaloblasts. (c) Megaloblasts with developing myeloid cells; the cell with a C-shaped nucleus (arrow) is a giant metamyelocyte. From Mehta and Hoffbrand (2009) Haematology at a Glance, 3rd edn. Wiley-Blackwell, Oxford.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


