17
Gram-Positive Rods
CHAPTER CONTENTS
INTRODUCTION
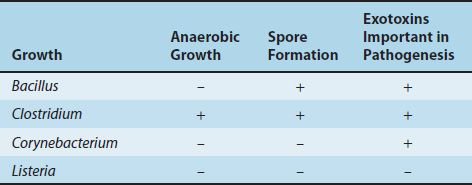
There are four medically important genera of gram-positive rods: Bacillus, Clostridium, Corynebacterium, and Listeria. Bacillus and Clostridium form spores, whereas Corynebacterium and Listeria do not. Members of the genus Bacillus are aerobic, whereas those of the genus Clostridium are anaerobic (Table 17–1).
These gram-positive rods can also be distinguished based on their appearance on Gram stain. Bacillus and Clostridium species are longer and more deeply staining than Corynebacterium and Listeria species. Corynebacterium species are club-shaped (i.e., they are thinner on one end than the other). Corynebacterium and Listeria species characteristically appear as V- or L-shaped rods.
SPORE-FORMING GRAM-POSITIVE RODS
BACILLUS
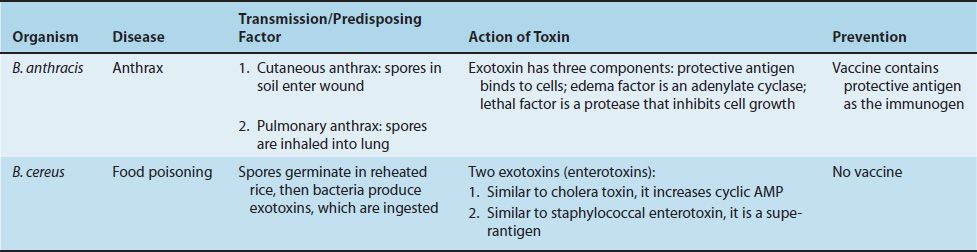
There are two medically important Bacillus species: Bacillus anthracis and Bacillus cereus. Important features of pathogenesis by these two Bacillus species are described in Table 17–2.
1. Bacillus anthracis
Disease
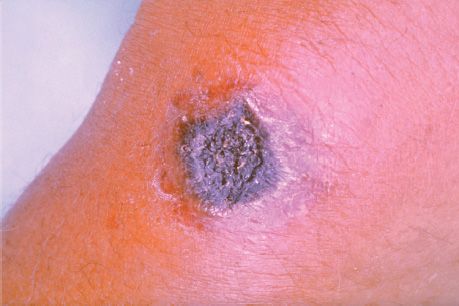
B. anthracis causes anthrax (Figure 17–1), which is common in animals but rare in humans. Human disease occurs in three main forms: cutaneous, pulmonary (inhalation), and gastrointestinal. In 2001, an outbreak of both inhalation and cutaneous anthrax occurred in the United States. The outbreak was caused by sending spores of the organism through the mail. There were 18 cases, causing 5 deaths in this outbreak.
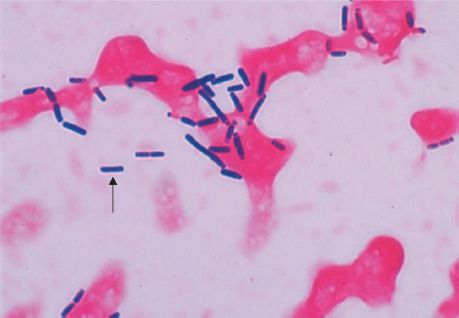
FIGURE 17–1 Skin lesion of anthrax. Note the black eschar, a necrotic lesion covered by a crust, caused by lethal factor, an exotoxin produced by Bacillus anthracis. Note the area of edema surrounding the eschar, which is caused by another exotoxin called edema factor. (Source: Centers for Disease Control and Prevention. CDC # 2033. CDC Provider: Dr. James H. Steele.)
Important Properties
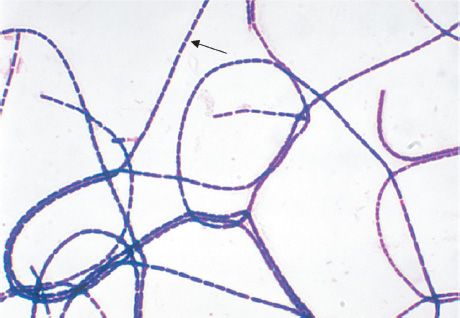
B. anthracis is a large gram-positive rod with square ends, frequently found in chains (Figure 17–2). Its antiphagocytic capsule is composed of D-glutamate. (This is unique—capsules of other bacteria are polysaccharides.) It is nonmotile, whereas other members of the genus are motile. Anthrax toxin is encoded on one plasmid, and the polyglutamate capsule is encoded on a different plasmid.
FIGURE 17–2 Bacillus anthracis—Gram stain. Arrow points to one large “box car–like” gram-positive rod within a long chain. (Figure courtesy of Public Health Image Library, Centers for Disease Control and Prevention.)
Transmission
Spores of the organism persist in soil for years. Humans are most often infected cutaneously at the time of trauma to the skin, which allows the spores on animal products, such as hides, bristles, and wool, to enter. Spores can also be inhaled into the respiratory tract. Pulmonary (inhalation) anthrax occurs when spores are inhaled into the lungs. Gastrointestinal anthrax occurs when contaminated meat is ingested.
Inhalation anthrax is not communicable from person to person, despite the severity of the infection. After being inhaled into the lung, the organism moves rapidly to the mediastinal lymph nodes, where it causes hemorrhagic mediastinitis. Because it leaves the lung so rapidly, it is not transmitted by the respiratory route to others.
Pathogenesis
Pathogenesis is based primarily on the production of two exotoxins, collectively known as anthrax toxin. The two exotoxins, edema factor and lethal factor, each consist of two proteins in an A–B subunit configuration. The B, or binding, subunit in each of the two exotoxins is protective antigen. The A, or active, subunit has enzymatic activity.
Edema factor, an exotoxin, is an adenylate cyclase that causes an increase in the intracellular concentration of cyclic adenosine monophosphate (AMP). This causes an outpouring of fluid from the cell into the extracellular space, which manifests as edema. (Note the similarity of action to that of cholera toxin.) Lethal factor is a protease that cleaves the phosphokinase that activates the mitogen-activated protein kinase (MAPK) signal transduction pathway. This pathway controls the growth of human cells, and cleavage of the phosphokinase inhibits cell growth. Protective antigen forms pores in the human cell membrane that allows edema factor and lethal factor to enter the cell. The name protective antigen refers to the fact that antibody against this protein protects against disease.
Clinical Findings
The typical lesion of cutaneous anthrax is a painless ulcer with a black eschar (crust, scab) (Figure 17–1). Local edema is striking. The lesion is called a malignant pustule. Untreated cases progress to bacteremia and death.
Pulmonary (inhalation) anthrax, also known as “wool-sorter’s disease,” begins with nonspecific respiratory tract symptoms resembling influenza, especially a dry cough and substernal pressure. This rapidly progresses to hemorrhagic mediastinitis, bloody pleural effusions, septic shock, and death. Although the lungs are infected, the classic features and X-ray picture of pneumonia are not present. Mediastinal widening seen on chest X-ray is an important diagnostic criterion. Hemorrhagic mediastinitis and hemorrhagic meningitis are severe life-threatening complications. The symptoms of gastrointestinal anthrax include vomiting, abdominal pain, and bloody diarrhea.
Laboratory Diagnosis
Smears show large, gram-positive rods in chains (Figure 17–2). Spores are usually not seen in smears of exudate because spores form when nutrients are insufficient, and nutrients are plentiful in infected tissue. Nonhemolytic colonies form on blood agar aerobically. In case of a bioterror attack, rapid diagnosis can be performed in special laboratories using polymerase chain reaction (PCR)–based assays. Another rapid diagnostic procedure is the direct fluorescent antibody test that detects antigens of the organism in the lesion. Serologic tests, such as an enzyme-linked immunosorbent assay (ELISA) test for antibodies, require acute and convalescent serum samples and can only be used to make a diagnosis retrospectively.
Treatment
Ciprofloxacin is the drug of choice. Doxycycline is an alternative drug. No resistant strains have been isolated clinically.
Prevention
Ciprofloxacin or doxycycline was used as prophylaxis in those exposed during the outbreak in the United States in 2001. People at high risk can be immunized with cell-free vaccine containing purified protective antigen as immunogen. The vaccine is weakly immunogenic, and six doses of vaccine over an 18-month period are given. Annual boosters are also given to maintain protection. Incinerating animals that die of anthrax, rather than burying them, will prevent the soil from becoming contaminated with spores.
2. Bacillus cereus
Disease
B. cereus causes food poisoning.
Transmission
Spores on grains such as rice survive steaming and rapid frying. The spores germinate when rice is kept warm for many hours (e.g., reheated fried rice). The portal of entry is the gastrointestinal tract.
Pathogenesis
B. cereus produces two enterotoxins. The mode of action of one of the enterotoxins is the same as that of cholera toxin (i.e., it adds adenosine diphosphate ribose, a process called ADP-ribosylation, to a G protein, which stimulates adenylate cyclase and leads to an increased concentration of cyclic AMP within the enterocyte). The mode of action of the other enterotoxin resembles that of staphylococcal enterotoxin (i.e., it is a superantigen).
Clinical Findings
There are two syndromes. (1) One syndrome has a short incubation period (4 hours) and consists primarily of nausea and vomiting, similar to staphylococcal food poisoning. (2) The other has a long incubation period (18 hours) and features watery, nonbloody diarrhea, resembling clostridial gastroenteritis.
Laboratory Diagnosis
This is not usually done.
Treatment
Only symptomatic treatment is given.
Prevention
There is no specific means of prevention. Rice should not be kept warm for long periods.
CLOSTRIDIUM
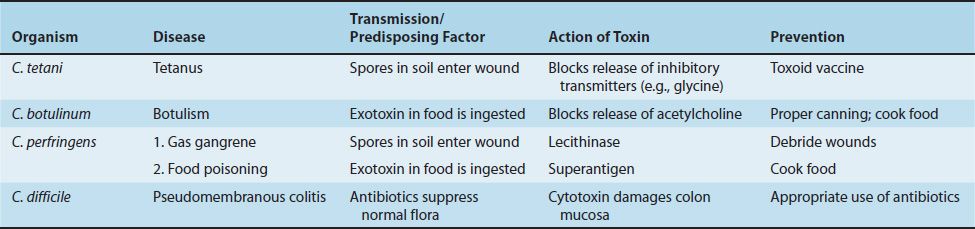
There are four medically important species: Clostridium tetani, Clostridium botulinum, Clostridium perfringens (which causes either gas gangrene or food poisoning), and Clostridium difficile. All clostridia are anaerobic, spore-forming, gram-positive rods (Figure 17–3). Important features of pathogenesis and prevention are described in Table 17–3.
FIGURE 17–3 Clostridium perfringens—Gram stain. Arrow points to a large gram-positive rod. (Used with permission from Professor Shirley Lowe, University of California, San Francisco School of Medicine.)
1. Clostridium tetani
Disease
C. tetani causes tetanus (Figure 17–4).
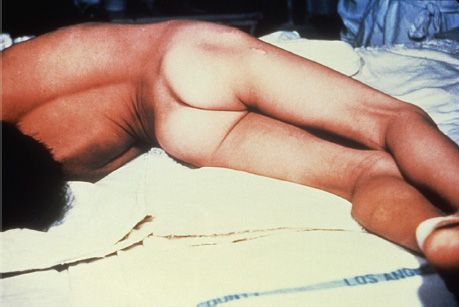
FIGURE 17–4 Tetanus. Note the marked hyperextension of the back, a position called opisthotonos, caused by tetanus toxin, an exotoxin that inhibits the release of mediators of the inhibitory neurons in the spinal cord. (Source: Centers for Disease Control and Prevention. CDC # 6373.)
Transmission
Spores are widespread in soil. The portal of entry is usually a wound site (e.g., where a nail penetrates the foot), but the spores can also be introduced during “skin-popping,” a technique used by drug addicts to inject drugs into the skin. Germination of spores is favored by necrotic tissue and poor blood supply in the wound. Neonatal tetanus, in which the organism enters through a contaminated umbilicus or circumcision wound, is a major problem in some developing countries.
Pathogenesis
Tetanus toxin (tetanospasmin) is an exotoxin produced by vegetative cells at the wound site. This polypeptide toxin is carried intra-axonally (retrograde) to the central nervous system, where it binds to ganglioside receptors and blocks release of inhibitory mediators (e.g., glycine and γ-aminobutyric acid [GABA]) at spinal synapses. Tetanus toxin and botulinum toxin (see later) are among the most toxic substances known. They are proteases that cleave the proteins involved in mediator release.
Tetanus toxin has one antigenic type, unlike botulinum toxin, which has eight. There is therefore only one antigenic type of tetanus toxoid in the vaccine against tetanus.
Clinical Findings
Tetanus is characterized by strong muscle spasms (spastic paralysis, tetany). Specific clinical features include lockjaw (trismus) due to rigid contraction of the jaw muscles, which prevents the mouth from opening; a characteristic grimace known as risus sardonicus; and exaggerated reflexes. Opisthotonos, a pronounced arching of the back due to spasm of the strong extensor muscles of the back, is often seen (Figure 17–4). Respiratory failure ensues. A high mortality rate is associated with this disease. Note that in tetanus, spastic paralysis (strong muscle contractions) occurs, whereas in botulism, flaccid paralysis (weak or absent muscle contractions) occurs.
Laboratory Diagnosis
There is no microbiologic or serologic diagnosis. Organisms are rarely isolated from the wound site. C. tetani produces a terminal spore (i.e., a spore at the end of the rod). This gives the organism the characteristic appearance of a “tennis racket.”
Treatment
Tetanus immune globulin (tetanus antitoxin) is used to neutralize the toxin. The role of antibiotics is uncertain. If antibiotics are used, either metronidazole or penicillin G can be given. An adequate airway must be maintained and respiratory support given. Benzodiazepines (e.g., diazepam [Valium]) should be given to prevent spasms.
Prevention
Tetanus is prevented by immunization with tetanus toxoid (formaldehyde-treated toxin) in childhood and every 10 years thereafter. Tetanus toxoid is usually given to children in combination with diphtheria toxoid and the acellular pertussis vaccine (DTaP).
When trauma occurs, the wound should be cleaned and debrided, and tetanus toxoid booster should be given. If the wound is grossly contaminated, tetanus immune globulin, as well as the toxoid booster, should be given and penicillin administered. Tetanus immune globulin (tetanus antitoxin) is made in humans to avoid serum sickness reactions that occur when antitoxin made in horses is used. The administration of both immune globulins and tetanus toxoid (at different sites in the body) is an example of passive–active immunity.
2. Clostridium botulinum
Disease
C. botulinum causes botulism.
Transmission
Spores, widespread in soil, contaminate vegetables and meats. When these foods are canned or vacuum-packed without adequate sterilization, spores survive and germinate in the anaerobic environment. Toxin is produced within the canned food and ingested preformed. The highest-risk foods are (1) alkaline vegetables such as green beans, peppers, and mushrooms and (2) smoked fish. The toxin is relatively heat-labile; it is inactivated by boiling for several minutes. Thus, disease can be prevented by sufficient cooking.
Pathogenesis
Botulinum toxin is absorbed from the gut and carried via the blood to peripheral nerve synapses, where it blocks release of acetylcholine. It is a protease that cleaves the proteins involved in acetylcholine release. The toxin is a polypeptide encoded by a lysogenic phage. Along with tetanus toxin, it is among the most toxic substances known. There are eight immunologic types of toxin; types A, B, and E are the most common in human illness. Botox is a commercial preparation of exotoxin A used to remove wrinkles on the face. Minute amounts of the toxin are effective in the treatment of certain spasmodic muscle disorders such as torticollis, “writer’s cramp,” and blepharospasm.
Clinical Findings
Descending weakness and paralysis, including diplopia, dysphagia, and respiratory muscle failure, are seen. No fever is present. In contrast, Guillain-Barré syndrome is an ascending paralysis (see Chapter 66).
Two special clinical forms occur: (1) wound botulism, in which spores contaminate a wound, germinate, and produce toxin at the site; and (2) infant botulism, in which the organisms grow in the gut and produce the toxin there. Ingestion of honey containing the organism is implicated in transmission of infant botulism. Affected infants develop weakness or paralysis and may need respiratory support but usually recover spontaneously. In the United States, infant botulism accounts for about half of the cases of botulism, and wound botulism is associated with drug abuse, especially skin-popping with black tar heroin.
Laboratory Diagnosis
The organism is usually not cultured. Botulinum toxin is demonstrable in uneaten food and the patient’s serum by mouse protection tests. Mice are inoculated with a sample of the clinical specimen and will die unless protected by antitoxin.
Treatment
Trivalent antitoxin (types A, B, and E) is given, along with respiratory support. The antitoxin is made in horses, and serum sickness occurs in about 15% of antiserum recipients.
Prevention
Proper sterilization of all canned and vacuum-packed foods is essential. Food must be adequately cooked to inactivate the toxin. Swollen cans must be discarded (clostridial proteolytic enzymes form gas, which swells cans).
3. Clostridium perfringens
C. perfringens causes two distinct diseases, gas gangrene and food poisoning, depending on the route of entry into the body.
Disease: Gas Gangrene
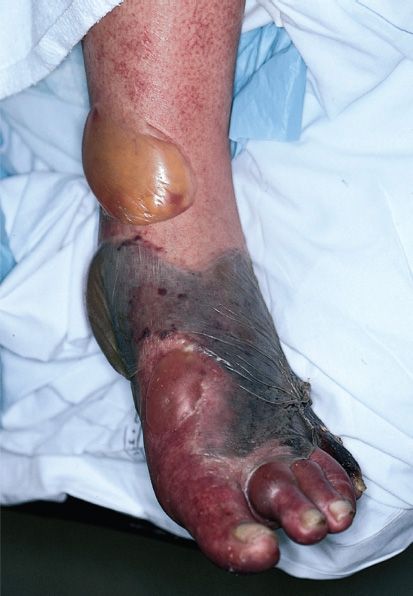
Gas gangrene (myonecrosis, necrotizing fasciitis) is one of the two diseases caused by C. perfringens (Figure 17–5). Gas gangrene is also caused by other histotoxic clostridia such as Clostridium histolyticum, Clostridium septicum, Clostridium novyi, and Clostridium sordellii. (C. sordellii also causes toxic shock syndrome in postpartum and postabortion women.)
FIGURE 17–5 Gas gangrene. Note large area of necrosis on lateral aspect of foot. Necrosis is mainly caused by lecithinase produced by Clostridium perfringens. Gas in tissue is a feature of gangrene produced by these anaerobic bacteria. A large gas- and fluid-filled bulla is seen near the ankle. (Used with permission from David Kaplan, MD.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree