18
Gram-Negative Rods Related to the Enteric Tract
CHAPTER CONTENTS
INTRODUCTION
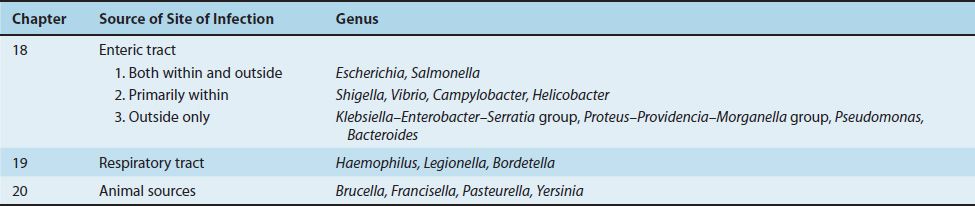
Gram-negative rods are a large group of diverse organisms (see Figures 18–1, 18–2, and 19–1). In this book, these bacteria are subdivided into three clinically relevant categories, each in a separate chapter, according to whether the organism is related primarily to the enteric or the respiratory tract or to animal sources (Table 18–1). Although this approach leads to some overlap, it should be helpful because it allows general concepts to be emphasized.
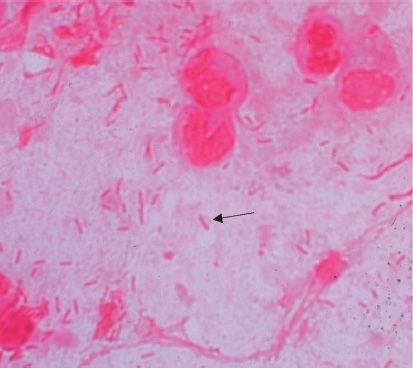
FIGURE 18–1 Escherichia coli—Gram stain. Arrow points to a gram-negative rod. (Used with permission from Professor Shirley Lowe, University of California, San Francisco School of Medicine.)
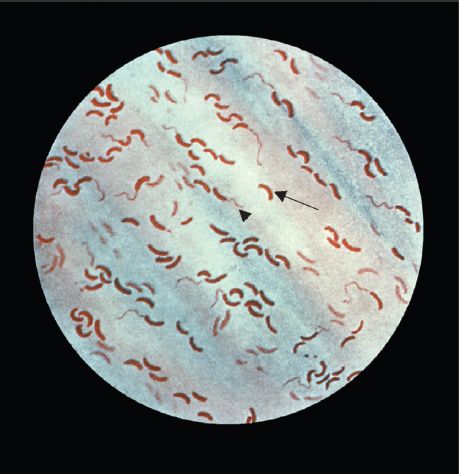
FIGURE 18–2 Vibrio cholerae—Gram stain. Long arrow points to a curved gram-negative rod. Arrowhead points to a flagellum at one end of a curved gram-negative rod. (Figure courtesy of Public Health Image Library, Centers for Disease Control and Prevention.)
Gram-negative rods related to the enteric tract include a large number of genera. These genera have therefore been divided into three groups depending on the major anatomic location of disease, namely, (1) pathogens both within and outside the enteric tract, (2) pathogens primarily within the enteric tract, and (3) pathogens outside the enteric tract (Table 18–1).
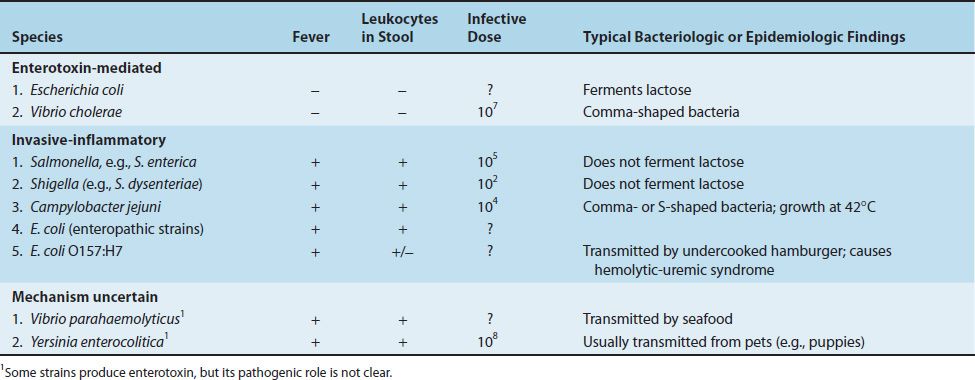
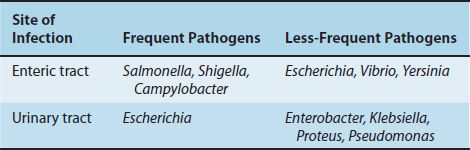
The frequency with which the organisms related to the enteric tract cause disease in the United States is shown in Table 18–2. Salmonella, Shigella, and Campylobacter are frequent pathogens in the gastrointestinal tract, whereas Escherichia, Vibrio, and Yersinia are less so. Enterotoxigenic strains of Escherichia coli are a common cause of diarrhea in developing countries but are less common in the United States. The medically important gram-negative rods that cause diarrhea are described in Table 18–3. Urinary tract infections are caused primarily by E. coli; the other organisms occur less commonly. The medically important gram-negative rods that cause urinary tract infections are described in Table 18–4.
TABLE 18–2 Frequency of Diseases Caused in the United States by Gram-Negative Rods Related to the Enteric Tract
Patients infected with such enteric pathogens as Shigella, Salmonella, Campylobacter, and Yersinia have a high incidence of certain autoimmune diseases such as Reiter’s syndrome (see Chapter 66). In addition, infection with Campylobacter jejuni predisposes to Guillain-Barré syndrome.
Before describing the specific organisms, it is appropriate to describe the family Enterobacteriaceae, to which many of these gram-negative rods belong.
ENTEROBACTERIACEAE & RELATED ORGANISMS
The Enterobacteriaceae is a large family of gram-negative rods found primarily in the colon of humans and other animals, many as part of the normal flora. These organisms are the major facultative anaerobes in the large intestine but are present in relatively small numbers compared with anaerobes such as Bacteroides. Although the members of the Enterobacteriaceae are classified together taxonomically, they cause a variety of diseases with different pathogenetic mechanisms. The organisms and some of the diseases they cause are listed in Table 18–5.
Features common to all members of this heterogeneous family are their anatomic location and the following four metabolic processes: (1) they are all facultative anaerobes; (2) they all ferment glucose (fermentation of other sugars varies); (3) none have cytochrome oxidase (i.e., they are oxidase-negative); and (4) they reduce nitrates to nitrites as part of their energy-generating processes.
These four reactions can be used to distinguish the Enterobacteriaceae from another medically significant group of organisms—the nonfermenting gram-negative rods, the most important of which is Pseudomonas aeruginosa.1
P. aeruginosa, a significant cause of urinary tract infection and sepsis in hospitalized patients, does not ferment glucose or reduce nitrates and is oxidase-positive. In contrast to the Enterobacteriaceae, it is a strict aerobe and derives its energy from oxidation, not fermentation.
Pathogenesis
All members of the Enterobacteriaceae, being gram-negative, contain endotoxin in their cell walls. In addition, several exotoxins are produced (e.g., E. coli and Vibrio cholerae secrete exotoxins, called enterotoxins, that activate adenylate cyclase within the cells of the small intestine, causing diarrhea) (see Chapter 7).
Antigens
The antigens of several members of the Enterobacteriaceae, especially Salmonella and Shigella, are important; they are used for identification purposes both in the clinical laboratory and in epidemiologic investigations. The three surface antigens are as follows:
(1) The cell wall antigen (also known as the somatic, or O, antigen) is the outer polysaccharide portion of the lipopolysaccharide (see Figure 2–6). The O antigen, which is composed of repeating oligosaccharides consisting of three or four sugars repeated 15 or 20 times, is the basis for the serologic typing of many enteric rods. The number of different O antigens is very large (e.g., there are approximately 1500 types of Salmonella and 150 types of E. coli).
(2) The H antigen is on the flagellar protein. Only flagellated organisms, such as Escherichia and Salmonella, have H antigens, whereas the nonmotile ones, such as Klebsiella and Shigella, do not. The H antigens of certain Salmonella species are unusual because the organisms can reversibly alternate between two types of H antigens called phase 1 and phase 2. The organisms may use this change in antigenicity to evade the immune response.
(3) The capsular or K polysaccharide antigen is particularly prominent in heavily encapsulated organisms such as Klebsiella. The K antigen is identified by the quellung (capsular swelling) reaction in the presence of specific antisera and is used to serotype E. coli and Salmonella typhi for epidemiologic purposes. In S. typhi, the cause of typhoid fever, it is called the Vi (or virulence) antigen.
Laboratory Diagnosis
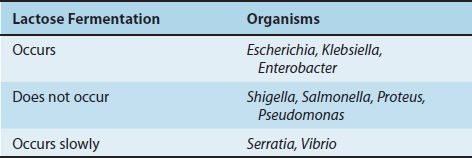
Specimens suspected of containing members of the Enterobacteriaceae and related organisms are usually inoculated onto two media, a blood agar plate and a selective differential medium such as MacConkey’s agar or eosin–methylene blue (EMB) agar. The differential ability of these latter media is based on lactose fermentation, which is the most important metabolic criterion used in the identification of these organisms (Table 18–6). On these media, the non–lactose fermenters (e.g., Salmonella and Shigella) form colorless colonies, whereas the lactose fermenters (e.g., E. coli) form colored colonies. On EMB agar, E. coli colonies have a characteristic green sheen. The selective effect of the media in suppressing unwanted gram-positive organisms is exerted by bile salts or bacteriostatic dyes in the agar.
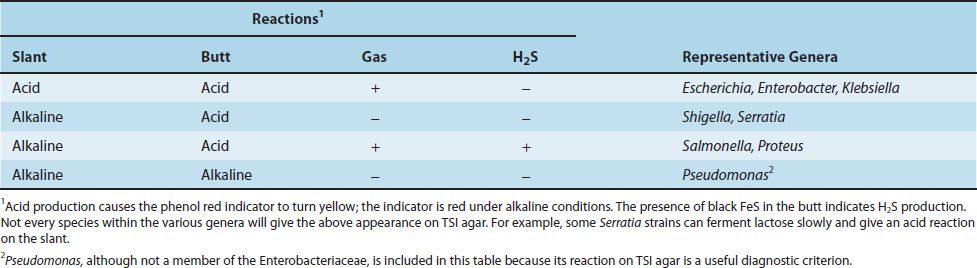
An additional set of screening tests, consisting of triple sugar iron (TSI) agar and urea agar, is performed prior to the definitive identification procedures. The rationale for the use of these media and the reactions of several important organisms are presented in the box titled “Agar Media for Enteric Gram-Negative Rods” (see later in the chapter) and in Table 18–7. The results of the screening process are often sufficient to identify the genus of an organism; however, an array of 20 or more biochemical tests is required to identify the species.
AGAR MEDIA FOR ENTERIC GRAM-NEGATIVE RODS
Another valuable piece of information used to identify some of these organisms is their motility, which is dependent on the presence of flagella. Proteus species are very motile and characteristically swarm over the blood agar plate, obscuring the colonies of other organisms. Motility is also an important diagnostic criterion in the differentiation of Enterobacter cloacae, which is motile, from Klebsiella pneumoniae, which is nonmotile.
If the results of the screening tests suggest the presence of a Salmonella or Shigella strain, an agglutination test can be used to identify the genus of the organism and to determine whether it is a member of group A, B, C, or D.
Coliforms & Public Health
Contamination of the public water supply system by sewage is detected by the presence of coliforms in the water. In a general sense, the term coliform includes not only E. coli, but also other inhabitants of the colon such as Enterobacter and Klebsiella. However, because only E. coli is exclusively a large intestine organism, whereas the others are found in the environment also, it is used as the indicator of fecal contamination. In water quality testing, E. coli is identified by its ability to ferment lactose with the production of acid and gas, its ability to grow at 44.5°C, and its characteristic colony type on EMB agar. An E. coli colony count above 4/dL in municipal drinking water is indicative of unacceptable fecal contamination. Because E. coli and the enteric pathogens are killed by chlorination of the drinking water, there is rarely a problem with meeting this standard. Disinfection of the public water supply is one of the most important advances of public health in the twentieth century.
Antibiotic Therapy
The appropriate treatment for infections caused by members of the Enterobacteriaceae and related organisms must be individually tailored to the antibiotic sensitivity of the organism. Generally speaking, a wide range of antimicrobial agents are potentially effective (e.g., some penicillins and cephalosporins, aminoglycosides, chloramphenicol, tetracyclines, quinolones, and sulfonamides). The specific choice usually depends on the results of antibiotic sensitivity tests.
Note that many isolates of these enteric gram-negative rods are highly antibiotic resistant because of the production of β-lactamases and other drug-modifying enzymes. These organisms undergo conjugation frequently, at which time they acquire plasmids (R factors) that mediate multiple drug resistance. For example, plasmid-encoded New Delhi metallo-β-lactamase causes resistance to penicillins, cephalosporins, monobactams, and carbapenems.
PATHOGENS BOTH WITHIN & OUTSIDE THE ENTERIC TRACT
ESCHERICHIA
Diseases
E. coli is the most common cause of urinary tract infection and gram-negative rod sepsis. It is one of the two important causes of neonatal meningitis and the agent most frequently associated with “traveler’s diarrhea,” a watery diarrhea. Some strains of E. coli are enterohemorrhagic and cause bloody diarrhea.
Important Properties
E. coli is a straight gram-negative rod (Figure 18–1), in contrast to the curved gram-negative rods of the genera Vibrio, Campylobacter, and Helicobacter.
E. coli is the most abundant facultative anaerobe in the colon and feces. It is, however, greatly outnumbered by the obligate anaerobes such as Bacteroides.
E. coli ferments lactose, a property that distinguishes it from the two major intestinal pathogens, Shigella and Salmonella. It has three antigens that are used to identify the organism in epidemiologic investigations: the O, or cell wall, antigen; the H, or flagellar, antigen; and the K, or capsular, antigen. Because there are more than 150 O, 50 H, and 90 K antigens, the various combinations result in more than 1000 antigenic types of E. coli. Specific serotypes are associated with certain diseases (e.g., O55 and O111 cause outbreaks of neonatal diarrhea).
Pathogenesis
The reservoir of E. coli includes both humans and animals. The source of the E. coli that causes urinary tract infections is the patient’s own colonic flora that colonizes the urogenital area. The source of the E. coli that causes neonatal meningitis is the mother’s birth canal; the infection is acquired during birth. In contrast, the E. coli that causes traveler’s diarrhea is acquired by ingestion of food or water contaminated with human feces. Note that the main reservoir of enterohemorrhagic E. coli O157 is cattle and the organism is acquired in undercooked meat.
E. coli has several clearly identified components that contribute to its ability to cause disease: pili, a capsule, endotoxin, and three exotoxins (enterotoxins), two that cause watery diarrhea and one that causes bloody diarrhea and hemolytic–uremic syndrome.
Intestinal Tract Infection
The first step is the adherence of the organism to the cells of the jejunum and ileum by means of pili that protrude from the bacterial surface. Once attached, the bacteria synthesize enterotoxins (exotoxins that act in the enteric tract), which act on the cells of the jejunum and ileum to cause diarrhea. The toxins are strikingly cell-specific; the cells of the colon are not susceptible, probably because they lack receptors for the toxin. Enterotoxigenic strains of E. coli can produce either or both of two enterotoxins.
(1) The heat-labile toxin (LT) acts by stimulating adenylate cyclase. Both LT and cholera toxin act by catalyzing the addition of adenosine diphosphate-ribose (a process called ADP-ribosylation) to the G protein that stimulates the cyclase. This irreversibly activates the cyclase. The resultant increase in intracellular cyclic adenosine monophosphate (AMP) concentration stimulates cyclic AMP–dependent protein kinase, which phosphorylates ion transporters in the membrane. The transporters export ions, which cause an outpouring of fluid, potassium, and chloride from the enterocytes into the lumen of the gut, resulting in watery diarrhea. Note that cholera toxin has the same mode of action.
(2) The other enterotoxin is a low-molecular-weight, heat-stable toxin (ST), which stimulates guanylate cyclase.
The enterotoxin-producing strains do not cause inflammation, do not invade the intestinal mucosa, and cause a watery, nonbloody diarrhea. However, certain strains of E. coli are enteropathic (enteroinvasive) and cause disease not by enterotoxin formation but by invasion of the epithelium of the large intestine, causing bloody diarrhea (dysentery) accompanied by inflammatory cells (neutrophils) in the stool.
Certain enterohemorrhagic strains of E. coli (i.e., those with the O157:H7 serotype) also cause bloody diarrhea by producing an exotoxin called Shiga toxin, so called because it is very similar to that produced by Shigella species. Shiga toxin acts by removing an adenine from the large (28S) ribosomal RNA, thereby stopping protein synthesis. Shiga toxin is encoded by temperate (lysogenic) bacteriophages. Shiga toxin is also called verotoxin because it has a cytopathic effect on Vero (monkey) cells in culture.
These O157:H7 strains are associated with outbreaks of bloody diarrhea following ingestion of undercooked hamburger, often at fast-food restaurants. The bacteria on the surface of the hamburger are killed by the cooking, but those in the interior, which is undercooked, survive. Also, direct contact with animals (e.g., visits to farms and petting zoos) has resulted in bloody diarrhea caused by O157:H7 strains.
Some patients with bloody diarrhea caused by O157:H7 strains also have a life-threatening complication called hemolytic–uremic syndrome (HUS), which occurs when Shiga toxin enters the bloodstream. This syndrome consists of hemolytic anemia, thrombocytopenia, and acute renal failure. The hemolytic anemia and renal failure occur because there are receptors for Shiga toxin on the surface of the endothelium of small blood vessels and on the surface of kidney epithelium. Death of the endothelial cells of small blood vessels results in a microangiopathic hemolytic anemia in which the red cells passing through the damaged area become grossly distorted (schistocytes) and then lyse. Thrombocytopenia occurs because platelets adhere to the damaged endothelial surface. Death of the kidney epithelial cells leads to renal failure. Treatment of diarrhea caused by O157:H7 strains with antibiotics, such as ciprofloxacin, increases the risk of developing HUS by increasing the amount of Shiga toxin released by the dying bacteria.
Urinary Tract Infections
Certain O serotypes of E. coli preferentially cause urinary tract infections. These uropathic strains are characterized by pili with adhesin proteins that bind to specific receptors on the urinary tract epithelium. The binding site on these receptors consists of dimers of galactose (Gal-Gal dimers). Cranberry juice contains flavonoids that inhibit the binding of pili to receptors and may be useful in the prevention of recurrent urinary tract infections. The motility of E. coli may aid its ability to ascend the urethra into the bladder and ascend the ureter into the kidney.
Systemic Infection
The other two structural components, the capsule and the endotoxin, play a more prominent role in the pathogenesis of systemic, rather than intestinal tract, disease. The capsular polysaccharide interferes with phagocytosis, thereby enhancing the organism’s ability to cause infections in various organs. For example, E. coli strains that cause neonatal meningitis usually have a specific capsular type called the K1 antigen. The endotoxin of E. coli is the cell wall lipopolysaccharide, which causes several features of gram-negative sepsis such as fever, hypotension, and disseminated intravascular coagulation.
Th-17 helper T cells that produce interleukin-17 are an important host defense against sepsis caused by enteric bacteria such as E. coli and Klebsiella. Patients infected with human immunodeficiency virus (HIV) experience a loss of Th-17 cells and are predisposed to sepsis caused by E. coli and Klebsiella.
Clinical Findings
E. coli causes a variety of diseases both within and outside the intestinal tract. The main clinical findings, the major pathogenetic factors, and the main laboratory results are described in Table 18–8.
(1) Clinical findings within the intestinal tract:
Diarrhea caused by enterotoxigenic E. coli is usually watery, nonbloody, self-limited, and of short duration (1–3 days). It is frequently associated with travel (traveler’s diarrhea, or “turista”).2
Infection with enterohemorrhagic E. coli (EHEC), on the other hand, results in a dysentery-like syndrome characterized by bloody diarrhea, abdominal cramping, and fever similar to that caused by Shigella.
The O157:H7 strains of E. coli also cause bloody diarrhea, which can be complicated by HUS. This syndrome is characterized by kidney failure, hemolytic anemia, and thrombocytopenia. The hemolytic anemia is caused by exotoxin-induced capillary damage, which results in damage to the red cells as they pass through the capillaries. These distorted, fragmented red cells called schistocytes can be seen on blood smear and are characteristic of a microangiopathic hemolytic anemia. In 2011, an outbreak of diarrhea and HUS in Germany was caused by a Shiga toxin–producing strain of E. coli that was typed as O104:H4, not O157:H7.
HUS occurs particularly in children who have been treated with fluoroquinolones or other antibiotics for their diarrhea. For this reason, antibiotics should not be used to treat diarrhea caused by EHEC.
(2) Clinical findings outside of the intestinal tract:
E. coli is the leading cause of community-acquired urinary tract infections. These infections occur primarily in women; this finding is attributed to three features that facilitate ascending infection into the bladder, namely, a short urethra, the proximity of the urethra to the anus, and colonization of the vagina by members of the fecal flora. It is also the most frequent cause of nosocomial (hospital-acquired) urinary tract infections, which occur equally frequently in both men and women and are associated with the use of indwelling urinary catheters. Urinary tract infections can be limited to the bladder or extend up the collecting system to the kidneys. If only the bladder is involved, the disease is called cystitis, whereas infection of the kidney is called pyelonephritis. The most prominent symptoms of cystitis are pain (dysuria) and frequency of urination; patients are usually afebrile. Pyelonephritis is characterized by fever, flank pain, and costovertebral angle tenderness; dysuria and frequency may or may not occur.
E. coli is also a major cause, along with the group B streptococci, of meningitis and sepsis in neonates. Exposure of the newborn to E. coli and group B streptococci occurs during birth as a result of colonization of the vagina by these organisms in approximately 25% of pregnant women. E. coli is the organism isolated most frequently from patients with hospital-acquired sepsis, which arises primarily from urinary, biliary, or peritoneal infections. Peritonitis is usually a mixed infection caused by E. coli or other facultative enteric gram-negative rod plus anaerobic members of the colonic flora such as Bacteroides and Fusobacterium.
Laboratory Diagnosis
Specimens suspected of containing enteric gram-negative rods, such as E. coli, are grown initially on a blood agar plate and on a differential medium, such as EMB agar or MacConkey’s agar. E. coli, which ferments lactose, forms pink colonies, whereas lactose-negative organisms are colorless. On EMB agar, E. coli colonies have a characteristic green sheen. Some of the important features that help distinguish E. coli from other lactose-fermenting gram-negative rods are as follows: (1) it produces indole from tryptophan, (2) it decarboxylates lysine, (3) it uses acetate as its only source of carbon, and (4) it is motile. E. coli O157:H7 does not ferment sorbitol, which serves as an important criterion that distinguishes it from other strains of E. coli. The isolation of enterotoxigenic or enteropathogenic E. coli from patients with diarrhea is not a routine diagnostic procedure.
Treatment
Treatment of E. coli infections depends on the site of disease and the resistance pattern of the specific isolate. For example, an uncomplicated lower urinary tract infection (cystitis) can be treated using oral trimethoprim-sulfamethoxazole or nitrofurantoin. Pyelonephritis can be treated with ciprofloxacin or ceftriaxone. However, E. coli sepsis requires treatment with parenteral antibiotics (e.g., a third-generation cephalosporin, such as cefotaxime, with or without an aminoglycoside, such as gentamicin). For the treatment of neonatal meningitis, a combination of ampicillin and cefotaxime is usually given. Antibiotic therapy is usually not indicated in E. coli diarrheal diseases. However, administration of trimethoprim-sulfamethoxazole or loperamide (Imodium) may shorten the duration of symptoms. Rehydration is typically all that is necessary in this self-limited disease.
Prevention
There is no specific prevention for E. coli infections, such as active or passive immunization. However, various general measures can be taken to prevent certain infections caused by E. coli and other organisms. For example, the incidence of urinary tract infections can be lowered by the judicious use and prompt withdrawal of catheters and, in recurrent infections, by prolonged prophylaxis with urinary antiseptic drugs (e.g., nitrofurantoin or trimethoprim-sulfamethoxazole). The use of cranberry juice to prevent recurrent urinary tract infections appears to be based on the ability of flavonoids in the juice to inhibit the binding of the pili of the uropathic strains of E. coli to the bladder epithelium rather than to acidification of the urine, which was the previous explanation.
Some cases of sepsis can be prevented by prompt removal of or switching the site of intravenous lines. Traveler’s diarrhea can sometimes be prevented by the prophylactic use of doxycycline, ciprofloxacin, trimethoprim-sulfamethoxazole, or Pepto-Bismol. Ingestion of uncooked foods and unpurified water should be avoided while traveling in certain countries.
SALMONELLA
Diseases
Salmonella species cause enterocolitis, enteric fevers such as typhoid fever, and septicemia with metastatic infections such as osteomyelitis. They are one of the most common causes of bacterial enterocolitis in the United States.
Important Properties
Salmonellae are gram-negative rods that do not ferment lactose but do produce H2S—features that are used in their laboratory identification. Their antigens—cell wall O, flagellar H, and capsular Vi (virulence)—are important for taxonomic and epidemiologic purposes. The O antigens, which are the outer polysaccharides of the cell wall, are used to subdivide the salmonellae into groups A–I. There are two forms of the H antigens, phases 1 and 2. Only one of the two H proteins is synthesized at any one time, depending on which gene sequence is in the correct alignment for transcription into mRNA. The Vi antigens (capsular polysaccharides) are antiphagocytic and are an important virulence factor for S. typhi, the agent of typhoid fever. The Vi antigens are also used for the serotyping of S. typhi in the clinical laboratory.
There are three methods for naming the salmonellae. Ewing divides the genus into three species: S. typhi, Salmonella choleraesuis, and Salmonella enteritidis. In this scheme there is one serotype in each of the first two species and 1500 serotypes in the third. Kaufman and White assign different species names to each serotype; there are roughly 1500 different species, usually named for the city in which they were isolated. Salmonella dublin according to Kaufman and White would be S. enteritidis serotype dublin according to Ewing. The third approach to naming the salmonellae is based on relatedness determined by DNA hybridization analysis. In this scheme, S. typhi is not a distinct species but is classified as Salmonella enterica serotype (or serovar) typhi. All three of these naming systems are in current use.
Clinically, the Salmonella species are often thought of in two distinct categories, namely, the typhoidal species (i.e., those that cause typhoid fever) and the nontyphoidal species (i.e., those that cause diarrhea [enterocolitis] and metastatic infections, such as osteomyelitis). The typhoidal species are S. typhi and S. paratyphi. The nontyphoidal species are the many serotypes of S. enterica. Of the serotypes, S. enterica serotype choleraesuis is the species most often involved in metastatic infections.
Pathogenesis & Epidemiology
The three types of Salmonella infections (enterocolitis, enteric fevers, and septicemia) have different pathogenic features.
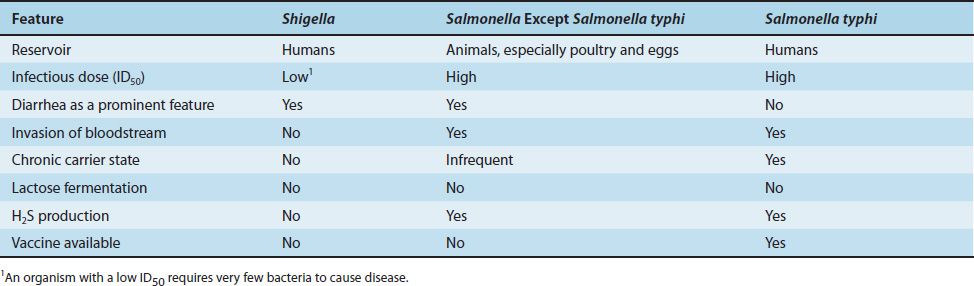
(1) Enterocolitis is characterized by an invasion of the epithelial and subepithelial tissue of the small and large intestines. Strains that do not invade do not cause disease. The organisms penetrate both through and between the mucosal cells into the lamina propria, with resulting inflammation and diarrhea. Neutrophils limit the infection to the gut and the adjacent mesenteric lymph nodes; bacteremia is infrequent in enterocolitis. In contrast to Shigella enterocolitis, in which the infectious dose is very small (on the order of 100 organisms), the dose of Salmonella required is much higher, at least 100,000 organisms. Various properties of salmonellae and shigellae are compared in Table 18–9. Gastric acid is an important host defense; gastrectomy or use of antacids lowers the infectious dose significantly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree