OVERVIEW OF TROPHOBLASTIC SUBPOPULATIONS AND PATHOGENESIS OF TROPHOBLASTIC NEOPLASIA
Trophoblast plays a crucial role in implantation and placentation. It exhibits a number of unique properties in accordance with its wide range of metabolic, endocrine, and invasive functions. Prior to the development of villi, the primitive trophoblast is termed previllous trophoblast, which may exhibit trophoblast stem cell features. Our knowledge of the previllous trophoblast is limited because few specimens are available for study. After formation of villi at approximately 2 weeks of gestational age, trophoblastic cells on chorionic villi are termed villous trophoblast, whereas trophoblastic cells in all other locations are designated extravillous trophoblast. The trophoblast in villous and extravillous locations can be broadly divided into three distinct populations based on morphologic, immunophenotypic, and functional studies. The cytotrophoblast is a relatively small, mononucleate cell with a distinct cell border that contains a small amount of eosinophilic cytoplasm (Fig. 49.1). In early gestation, the cytotrophoblast on the villous surface differentiates along two main pathways, developing into either a villous or extravillous trophoblastic lineage (2,3). On the villous surface, cytotrophoblastic cells fuse to form syncytiotrophoblastic cells, which are terminally differentiated cells. They synthesize and secrete a variety of pregnancy-associated hormones including human chorionic gonadotropin (hCG) and human placental lactogen (hPL). The syncytiotrophoblast is multinucleated, and often, the nuclei appear dark and pyknotic. There are numerous microvilli carpeting the syncytiotrophoblastic surface to increase the surface area and to facilitate molecular exchange and transportation. The cytoplasm of syncytiotrophoblastic cells is dense and stains deeply eosinophilic to basophilic (Fig. 49.1). Often, there are multiple intracytoplasmic vacuoles that result from dilatation of the endoplasmic reticulum and lacunae formed by complex infoldings of the plasma membrane. In contrast, cytotrophoblastic cells at the margin of what are destined to become anchoring villi display a morphologic spectrum of differentiation resulting in the development of intermediate trophoblastic cells within the trophoblastic columns (Fig. 49.1). This differentiation from cytotrophoblast to intermediate trophoblast is accompanied by gradual enlargement of the cells and their nuclei, accompanied by cytoplasmic clearing. From the trophoblastic columns, intermediate trophoblastic cells infiltrate the decidua and myometrium and invade and replace the spiral arteries of the basal plate to establish the maternal/fetal circulation. The differentiation of intermediate trophoblast toward the distal ends of the anchoring villi and in the endomyometrium is accompanied by a decrease in cellular proliferation (4). The term intermediate trophoblast is interchangeable with extravillous trophoblast in most of the literature.
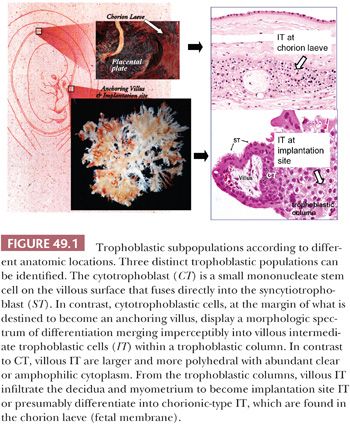
The morphologic features of intermediate trophoblastic cells are quite variable depending on their anatomic locations. In the trophoblastic columns, the intermediate trophoblastic cells are mononucleate, larger than cytotrophoblastic cells, and polygonal with clear cytoplasm. We have termed the intermediate trophoblastic cells that infiltrate the endomyometrium of the placental site the implantation site intermediate trophoblastic cells. In the endometrium, these cells are polygonal and contain abundant amphophilic cytoplasm closely resembling the decidual cells with which they are admixed, but their hyperchromatic nuclei with irregular outlines and frequent deep folds or clefts distinguish them from decidual cells. In the myometrium, implantation site intermediate trophoblastic cells are frequently spindle-shaped and resemble the surrounding smooth muscle cells. Occasionally, mononucleate intermediate trophoblastic cells in the implantation site fuse to form multinucleated intermediate trophoblastic cells (so-called syncytiotrophoblastic giant cells) (4). Last, in the chorion laeve, there are two distinct populations of cells: one containing eosinophilic and the other containing clear cytoplasm (5). Based on previous immunostaining studies (3,6), we believe these cells represent a subpopulation of intermediate trophoblastic cells rather than a subtype of cytotrophoblastic cells and prefer the designation chorionic-type intermediate trophoblastic cells for both the cells with clear and eosinophilic cytoplasm. The functional role of chorionic-type intermediate trophoblast is not known. Thus, at present, we subdivide intermediate trophoblast into three types based on anatomic location: intermediate trophoblast in the trophoblastic column, implantation site intermediate trophoblast, and chorionic-type intermediate trophoblast. The various trophoblastic subpopulations have different immunophenotypes (1,7) (Table 49.2). Identification and characterization of trophoblast-associated markers provide not only new insights into the physiology of different trophoblastic subpopulations but also immunohistochemical markers for the differential diagnosis in pathology practice.
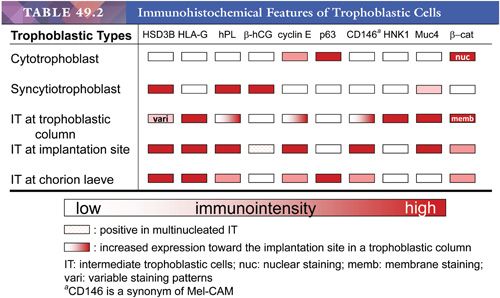
The recognition of trophoblastic subpopulations and their unique gene expression profiles (Table 49.2) also helps to understand the pathogenesis of various gestational trophoblastic lesions. Molecular analysis on gestational trophoblastic neoplasms is largely based on the characterization of gene expression profiles in various types of tumors and correlation of unique gene expression patterns to different trophoblastic subpopulations in normal early placentas (1,3). It has been proposed that following neoplastic transformation of trophoblastic stem cells, presumably the cytotrophoblasts, specific differentiation programs dictate the type of trophoblastic tumor to develop. These patterns of differentiation in gestational trophoblastic neoplasms recapitulate the stages of early placental development. Thus, choriocarcinoma is composed of variable amounts of neoplastic cytotrophoblast, syncytiotrophoblast, and intermediate trophoblast and resembles the previllous blastocyst, which is composed of a similar mixture of trophoblastic subpopulations. In contrast, the neoplastic cytotrophoblast in placental site trophoblastic tumor (PSTT) differentiates mainly into intermediate trophoblastic cells in an implantation site, whereas the neoplastic cytotrophoblast in epithelioid trophoblastic tumor (ETT) differentiates into chorionic-type intermediate trophoblastic cells in chorion laeve. According to this model, choriocarcinoma is the most primitive trophoblastic tumor as compared to PSTT and ETT. This hypothesis explains the existence of gestational trophoblastic neoplasms with a mixed histologic feature, including choriocarcinoma and PSTT and/or ETT.
CLINICAL AND PATHOLOGIC FEATURES OF GESTATIONAL TROPHOBLASTIC DISEASE
HYDATIDIFORM MOLE
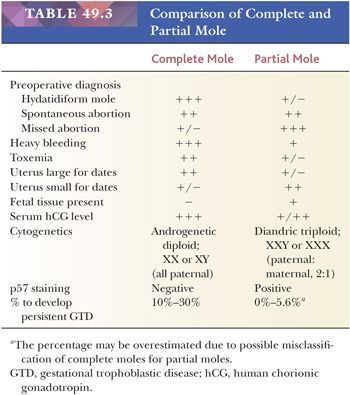
Hydatidiform mole, complete or partial, represents an abnormal placenta characterized by enlargement of chorionic villi caused by central edema of the stroma. Variable hyperplasia of the villous trophoblastic cells is present, and this hyperplasia may be marked. A complete mole is distinguished from a partial mole by the amount of villous involvement. The edema is generalized in a complete mole, whereas in a partial mole, the edematous change affects some of the villi. The hydropic villous changes may be related to retarded vasculogenic differentiation in villous stroma. Based on CD31 immunoreactivity, Kim et al. (8) have demonstrated a significant reduction of mature blood vessels with distinct lumens in the villous stroma in early-stage molar pregnancy, probably due to increased apoptosis in the precursor components of blood vessels. Thus, impairment of vasculogenesis may result in a lack of vascular drainage and cause progressive accumulation of vesicular fluid and lead to the formation of hydropic villi. In North America and Europe, the incidence of hydatidiform moles varies geographically and in general occurs in approximately 1 per 1000 pregnancies, although it can be as high as 1 in 600 viable conceptions in the United Kingdom (9). In some areas of Asia and the Middle East, an incidence rate of 1 to 10 per 1000 pregnancies has been reported (10). The ratio of complete versus partial moles varies among studies, and most reports show that complete moles outnumber partial moles (11,12). Clinical, pathologic, and cytogenetic differences separate the two forms of hydatidiform mole, yet all molar pregnancies may have the potential for persistent gestational trophoblastic disease (10), although the risk is significantly higher in complete than in partial moles.
Cytogenetic analysis of moles shows different mechanisms of their origins (10). The complete mole is diploid and is androgenetic, meaning that both genomic complements are of paternal origin. The majority of complete moles exhibit a karyotype of 46,XX and develop from the fertilization of an anuclear “empty” ovum by a single haploid (23X) sperm, in which the haploid genome duplicates (11). The remaining group (4% to 15%) of complete moles are also androgenetic but are formed by dispermy (i.e., fertilization of an ovum lacking functional maternal chromosomes by two spermatozoa). Partial moles are cytogenetically different, being triploid with two sets of chromosomes of paternal origin and a haploid maternal set in most cases. This triploid chromosomal composition in those specimens is known as diandric. Usually, the triploid set is XXY (58%). Less commonly, it is XXX (40%) or XYY (2%). Although partial moles are almost always triploids, products of conception with triploids may not necessarily be partial moles. The triploids are composed of different groups with distinct pathogenesis (13). For example, digynic placentas with one set of chromosomes of paternal origin and two sets of maternal origin do not exhibit histopathologic features that characterize diandric partial moles (14). In digynic triploids, fetal tissue and well-preserved fetal red blood cells in chorionic villi are more frequently identified. Although digynic triploids occasionally show irregular, dimorphic villi and a mild form of trophoblast hyperplasia, they do not exhibit hydropic degeneration and are rarely suspicious for partial moles (14).
In summary, a predominance of paternal chromosomes characterizes the cytogenetics of molar pregnancy. The ratio of paternal-to-maternal chromosomes is 2:0 in a complete mole and 2:1 in a partial mole.
COMPLETE MOLE
Morphologic Features
In well-developed cases, massively enlarged, edematous villi give the characteristic grapelike appearance to the placenta. A classical complete mole is often voluminous, consisting of up to 500 cc or more of bloody tissue. The hallmark of the complete mole is gross, generalized villous edema (Fig. 49.2). In a well-established complete mole, enlarged villi form grapelike, transparent vesicles measuring 1 to 2 cm across. When a complete mole is encountered in a hysterectomy specimen, the uterus is enlarged and molar vesicles protrude on opening. Only rarely is an embryo or fetus associated with a complete mole (15). In most of these cases, this represents a twin gestation (i.e., a fetus with a normal placenta and the second placenta a complete mole). Due to a widespread use of sonogram in prenatal care, most complete moles are now diagnosed in the first trimester. As a result, the specimen volume of early complete moles is significantly less than in the past (16), and the hydropic change may be subtle in those specimens, posing a challenge to their diagnosis.

The primary histologic feature of a complete mole is generalized hydropic villous change. Most villi are edematous (Fig. 49.3), and many have cisterns that are central, acellular, fluid-filled spaces. A small rim of mesenchyme separates the cistern from the surface layer of trophoblastic cells. Often, the border of the mesenchyme with the acellular cistern is abrupt and well defined. Necrosis and patchy calcification of villous stroma may be seen.
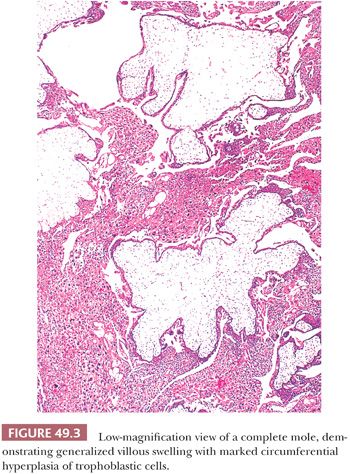
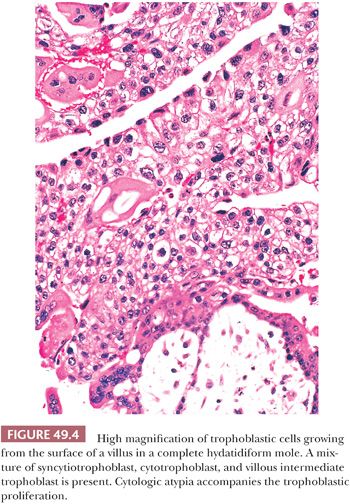
The other hallmark of a complete mole is proliferation of villous trophoblastic cells (Fig. 49.3). This proliferation is irregular, affecting villi unevenly; some, including smaller villi, may show marked overgrowth of trophoblast, whereas others, including larger villi, may show little trophoblastic hyperplasia. The proliferating trophoblastic elements, composed of syncytiotrophoblast, cytotrophoblast, and intermediate trophoblast, show a random, circumferential growth from the villous surface in marked contrast to the orderly growth of normal trophoblastic cells, showing proliferation from only the end of anchoring villi in early nonmolar placentas (so-called polarized villi). The trophoblastic cells of a mole, in addition to being hyperplastic, often show considerable cytologic atypia (Fig. 49.4) with nuclear and cytoplasmic enlargement, irregularity of nuclear outlines, and hyperchromasia. The amount of trophoblastic growth is highly variable in moles; it may be exuberant, or focal and minimal. Consequently, this feature does not have to be prominent to establish the diagnosis. Furthermore, the histologic grade of the trophoblastic cells in a complete mole has no apparent effect on the overall prognosis (17). In addition to the marked villous swelling and trophoblastic hyperplasia, a complete mole is typically characterized by an absence of fetal parts or amnion.
In addition to proliferation of trophoblast on the villous surface, hydatidiform moles are invariably associated with hyperplasia of intermediate trophoblastic cells in an implantation site, resembling an exaggerated placental site (see the following discussion) (18). The infiltrating implantation site intermediate trophoblastic cells are generally more numerous and more atypical than those in an exaggerated placental site not associated with a hydatidiform mole. Nonetheless, the immunophenotype of intermediate trophoblast in the molar implantation site is typical of implantation site intermediate trophoblastic cells found in normal gestation. The implantation site of a hydatidiform mole has an increased Ki-67 labeling index in contrast to a very low or absent labeling index in the exaggerated placental site of nonmolar gestation, suggesting that these two lesions are different despite their similar immunohistochemical features (18,19).
As noted previously, the typical morphologic features of a complete mole may be absent or very subtle in molar specimens evacuated in the first trimester. These complete moles (usually evacuated between 8 to 12 weeks) are referred to as “early complete moles.” The morphologic features of complete moles diagnosed at this “early” stage differ from those diagnosed in the second trimester. The histologic features of an early complete mole are (a) redundant or polypoid bulbous terminal villi, (b) hypercellular villous stroma with primitive stellate cells, (c) a labyrinthine network of villous stromal canaliculi, (d) focal hyperplasia of cytotrophoblast and syncytiotrophoblast on both villi and the undersurface of the chorionic plate, (e) atypical trophoblast lining the villi and in the implantation site (16,20), and (f) increased villous stromal apoptosis (8). It should be mindful that the mentioned morphologic criteria may not be specific and sensitive for the diagnosis of a (early) complete mole, and molecular study should be considered if the placental specimen is morphologically suspicious for a complete mole. Please see the section “Differential Diagnosis of Molar Placenta Based on Molecular and Immunohistochemical Assays” for molecular testing of molar placenta.
PARTIAL MOLE
Morphologic Features
The hallmark of a classical partial mole is a mixture of large, edematous villi and small, normal-sized villi without edema (Fig. 49.5). At least some of the hydropic villi show a central, acellular cistern like those seen in a complete mole. The small villi often show fibrosis. Trophoblastic hyperplasia is limited and focal and is often confined to the syncytiotrophoblast, forming syncytiotrophoblastic protrusions. As in a complete mole, the trophoblastic overgrowth, where present, is haphazard and circumferential over the surface of the villi. The villi often have irregular, scalloped outlines. These irregular outlines produce infoldings of trophoblastic cells into the villous stroma, which, when prominent and sectioned tangentially, appear to be inclusions of trophoblast within the stroma (Fig. 49.6). Evidence of a fetus with fetal parts or an amnion can be seen in some well-sampled cases. With fetal development, villous stromal vasculature often persists, and these vessels may contain nucleated erythrocytes.
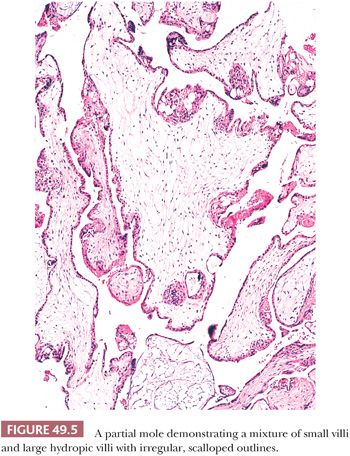
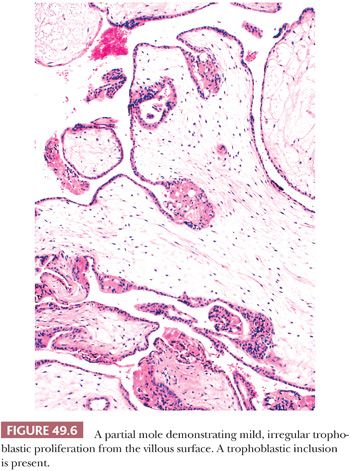
In summary, no single or combined morphologic features are sufficient to definitely diagnose a partial mole. It has been proposed that the presence of any one of the following histologic findings should prompt DNA genotyping to rule out a partial mole: two populations of villi, round or oval pseudoinclusions, cistern formation, and villous size of greater than or equal to 2.5 mm (21). Please see the section “Differential Diagnosis of Molar Placenta Based on Molecular and Immunohistochemical Assays” for molecular testing of molar placentas.
Differential Diagnosis of Molar Placenta Based on Morphology
The differential diagnosis of a hydatidiform mole, complete or partial, usually includes early nonmolar pregnancy including trisomy placentas. Morphologic distinction of a hydatidiform mole from an abortus with abnormal villous morphology may be problematic, and there is a considerable degree of interobserver variation in making this distinction (22–24). For example, chromosomal abnormalities that are not triploid, such as trisomy, may display hydropic change and mild trophoblastic hyperplasia that mimic a partial mole (24). These abnormal placentas associated with hydropic abortus are not associated with an increased risk of gestational trophoblastic disease and therefore the differential diagnosis is important. The villi in a hydropic abortus are enlarged only slightly and do not assume the large dimensions found in complete or partial moles. Cisterns can be seen in nonmolar abortions, but they are focally distributed. Hyperplasia of villous trophoblast is not evident in these cases. The proliferative trophoblast on the villous surface of early pregnancy also must be distinguished from the trophoblastic hyperplasia of molar pregnancy. The trophoblast proliferating from the villous surface of an abortus shows polar distribution characterized by proliferation of trophoblast at the distal end of the villus that implants into the basal plate. Similarly, villi with trophoblastic islands can be regarded as polarized villi that are also associated with a nonmolar abortus. This directional orientation contrasts with the irregular or circumferential proliferation of molar trophoblast. Moreover, the presence of gestational sac may serve as good evidence against a molar placenta. In conclusion, partial moles usually display at least three of the following histologic features: two discrete populations of villi, circumferential mild trophoblastic hyperplasia, trophoblastic inclusions, prominent scalloping of villi, or cistern formation. In contrast, nontriploid abortus displays at most two of the diagnostic features of partial moles (24).
Although molar pregnancies usually present with a moderate volume of tissue, not uncommonly, the amount of tissue is scant, and gross features helpful for anticipating the diagnosis of molar pregnancy are absent. This problem may be encountered when suction curettage causes collapse of villi onto the filter or gauze used to collect the specimen. Repeat curettage after initial evacuation of a hydatidiform mole may contain proliferating trophoblastic cells with a minimal amount of villi, raising the possibility of a postmolar choriocarcinoma. In general, choriocarcinoma cannot be diagnosed in the presence of villi (with an exception of the rare intraplacental choriocarcinoma), and the presence of proliferating trophoblastic cells in this setting most likely represents a persistent mole. However, neither invasive mole nor choriocarcinoma can be completely excluded, and meticulous follow-up with serum β-hCG titers is needed. Importantly, nonvillous trophoblastic elements after evacuation of a mole do not necessarily indicate the presence of choriocarcinoma. The diagnosis of a choriocarcinoma generally requires the absence of villi, along with evidence of destructive and infiltrative growth of tumor cells in the endomyometrium. Recently, the stromal apoptotic index has been shown to be significantly higher in very early complete mole than in very early partial mole or normal placental tissues, which can be potentially useful in differential diagnosis (8).
In practice, the most important diagnostic issue for pathologists who encounter specimens with abnormal villous morphology is to correctly diagnose complete moles because patients with complete moles have a higher risk of developing persistent gestational trophoblastic diseases than those with a partial mole and hydropic abortus. The differential diagnosis between complete mole and partial mole/hydropic abortus is primarily based on histologic features as previously mentioned. Several features distinguish a partial mole from a complete mole (reviewed in Genest [25]) (Table 49.3). First, the amount of tissue in a classical partial mole is generally less than in a complete mole. The gross specimen shows large and hydropic villi (like those seen in a complete mole) mixed with nonmolar placental tissue, an important distinguishing feature. In some cases of the partial mole, a fetus or fetal parts may be found and, when present, may show gross developmental abnormalities. Despite the gross and microscopic features that characterize complete mole, partial mole, and nonmolar hydropic abortus, it is challenging for pathologists to accurately diagnose them when the specimens are obtained in early gestation. It is not uncommon that an early complete mole is erroneously diagnosed as a partial mole and a nonmolar hydropic abortus as a partial mole.
Differential Diagnosis of Molar Placenta Based on Molecular and Immunohistochemical Assays
The limitation in differential diagnosis of molar versus nonmolar placentas and complete versus partial moles poses an unmet need to apply molecular tests for an accurate diagnosis. Because complete mole, partial mole, and nonmolar placenta have unique genetic constituents, analyzing their molecular genetic alterations could be informative and useful for pathologists to render a precise diagnosis as an adjunct to morphologic features. Indeed, recent reports have shown the value of ancillary molecular techniques, including immunohistochemistry using the p57 antibody and short tandem repeat (microsatellite marker) genotyping, in distinguishing hydatidiform moles from nonmolar specimens and for subtyping hydatidiform moles into complete moles and partial moles (26–31). In general, nonmolar specimens are p57-positive and biparental diploid (two copies of genetic complement from both mother and father); partial hydatidiform moles are p57-positive and diandric triploid (three copies of genetic complement, two from father and one from mother); and complete hydatidiform moles are p57-negative and androgenetic diploid (two copies of genetic complement both from father).
Immunohistochemical stains with the p57 antibody serve as the first-line assay in the diagnosis of complete moles. Expression of p57, a paternally imprinted maternally expressed gene, is absent in villous trophoblast and stromal cells in cases of complete moles (because they usually lack maternal alleles), including the early ones, in contrast to positive immunoreactivity in partial moles and nonmolar placentas such as hydropic abortus (30,32–36). It should not be overemphasized that assessment of p57 immunoreactivity should be performed only in villous trophoblast and stroma cells rather than intermediate trophoblastic cells in the endomyometrium as the latter may reexpress p57 due to a mechanism related to “epigenetic relaxation” in a complete mole. The accuracy of p57 staining in the diagnosis of complete moles has been recently validated (30,37). Although an undetectable p57 staining supports the diagnosis of a complete mole, p57 immunohistochemistry cannot be used to distinguish a partial mole from a nonmolar specimen because both express p57 (they contain maternal alleles). In this case, genotyping analysis using short tandem repeats (microsatellite markers) can be applied to determine the parental source of polymorphic alleles and ploidy in a molecular/genetic laboratory. The results showing androgenetic diploidy, diandric triploidy, and biparental diploidy are diagnostic of complete mole, partial mole, and nonmolar placenta (diploid hydropic abortus), respectively.
Clinicopathologic Correlation
In the past, a complete mole presented between the 11th and 25th week of pregnancy with an average gestational age of about 16 weeks. With the routine use of ultrasound during pregnancy, complete moles are increasingly being diagnosed earlier; therefore, many of the classic signs and symptoms as discussed in the following texts are absent or very subtle. In a classical case, excessive uterine enlargement is common and is often accompanied by other symptoms such as severe vomiting (hyperemesis gravidarum) and toxemia of pregnancy. Toxemia associated with molar pregnancy may be a clinical clue to the diagnosis because this disorder generally occurs later in gestation (third trimester) with nonmolar pregnancies. Often, patients with complete mole spontaneously abort, presenting with vaginal bleeding or passage of molar vesicles. Ovarian enlargement due to multiple theca lutein cysts (hyperreactio luteinalis) occurs in some patients with a complete mole. The β-hCG level is markedly elevated. Ultrasonography often discloses a characteristic “snowstorm” appearance in a well-developed case.
In patients with a classical partial mole, the duration of pregnancy tends to be greater than in patients with a complete mole, with an average gestational age of about 19 weeks (38,39) (Table 49.3). Often, the gestational age is greater than 20 weeks. Usually, the patient presents with abnormal uterine bleeding and clinically appears to have a spontaneous or missed abortion (38,39). As with complete moles, patients with a partial mole are now diagnosed earlier because of the routine use of ultrasound in pregnancy. As a result, some of the clinical differences between complete and partial moles are less clearly defined. In patients with a partial mole, the uterus is typically normal or small in size for the gestational age, and serum β-hCG levels generally do not show the marked elevation seen in a complete mole (40). Toxemia of pregnancy also may occur in association with a partial mole.
Behavior and Treatment
Many of the long-term follow-up studies of hydatidiform moles were reported before partial moles were recognized as a distinctive variant, so comprehensive comparison of the clinicopathologic features for these two forms of mole is not always feasible. Nonetheless, available data indicate that 10% to 30% of complete moles result in persistent gestational trophoblastic diseases, as evidenced by a plateau or rise in β-hCG titers or the presence of extrauterine disease (39,41,42). Of note, persistent gestational trophoblastic disease represents a clinical entity rather than a histopathologic diagnosis. Invasive moles likely represent the majority of persistent gestational trophoblastic diseases, and among them, choriocarcinoma is the most serious form, occurring about 2% to 3% of patients with complete moles (43).
Recent studies have demonstrated that as compared to complete moles, patients with partial moles have a lower risk of developing subsequent persistent or metastatic gestational trophoblastic disease with risk estimates of 0% to 5.6% (12,41,44–49). Misdiagnosis of early complete moles as partial moles may contribute to the overestimated frequency in some of the previous studies (50). There are few well-documented reports of choriocarcinoma following a partial mole (51). Reliable prognostic features of molar pregnancy have not been found. Specifically, histologic grading and expression of proliferating cell nuclear antigen (PCNA) and Ki-67 do not correlate with clinical outcome (52).
Because of the risk of persistent gestational trophoblastic disease, close monitoring of serum β-hCG levels is necessary following the diagnosis of any form of hydatidiform mole until levels fall to and remain in the normal range (42). A chest radiograph after diagnosis is useful for detecting early pulmonary lesions as well as providing a baseline should pulmonary disease ensue. Modern chemotherapy has transformed the prognosis of persistent trophoblastic disease, and more than 90% of patients with persistent gestational trophoblastic disease following molar pregnancy can be cured or achieve long-term remission (42,53). In general, normal subsequent pregnancy outcomes can be anticipated in patients with molar pregnancy after treatment (54).
INVASIVE MOLE
An invasive hydatidiform mole is almost invariably a sequela of a complete hydatidiform mole or less likely a partial mole. The pathologic diagnosis is based on the presence of molar villi with associated trophoblastic cells in the myometrium and broad ligament or at distant sites, almost always the vagina, vulva, or lung. A clinical diagnosis of invasive mole can be suspected when β-hCG titers plateau or rise following evacuation of a mole, but because choriocarcinoma can also supervene in this setting, the clinical diagnosis should be persistent GTD. The exact frequency of invasive mole is difficult to determine because of the lack of pathologic confirmation in most cases. Best estimates are that invasive mole is a clinically significant sequela in about 15% to 20% of patients with complete moles.
Pathologic Features
Grossly, invasive mole in the uterus results in an irregular, often hemorrhagic lesion that penetrates into the myometrium. The lesion can grow through the myometrium, perforating the serosa or extending into the broad ligament and adnexa. Extracavitary hydropic villi are grossly visible in some cases, but often they are difficult to detect.
Microscopically, invasive mole has molar villi with associated trophoblastic cells, either within the myometrium, within myometrial blood vessels, or at distant sites. The villi are enlarged but often are not as large as those of a typical complete intrauterine mole. The amount of trophoblastic proliferation around the villi of invasive mole is highly variable; there may be marked proliferation that obscures the villi. In such cases, careful sectioning and scrutiny for villi is necessary to avoid misclassification of the lesion as choriocarcinoma.
Differential Diagnosis
The differential diagnosis of invasive mole includes intracavitary, noninvasive hydatidiform mole; choriocarcinoma; and placenta increta/percreta. The pathologic diagnosis of invasive mole requires clear-cut evidence of extension of molar tissue into the myometrium and its vessels or deportation to distant sites. Marked trophoblastic proliferation associated with intracavitary hydatidiform mole does not necessarily indicate the presence of an invasive mole. An invasive mole may be difficult to distinguish from choriocarcinoma, as both entities feature a proliferation of trophoblastic cells in the myometrium, vagina, or lungs. These two disorders are distinguished by the presence or absence of chorionic villi.
Placenta increta and percreta represent growth of nonmolar placental tissue into the myometrium. These abnormal placentas lack the villous hydrops and abnormal trophoblastic proliferation found in invasive mole, and they almost always present during parturition when the placenta fails to separate from the myometrium. This presentation contrasts with an invasive mole, which usually is a sequela of an intracavitary hydatidiform mole.
Behavior and Treatment
Invasive hydatidiform mole may cause severe, life-threatening hemorrhage if it perforates the uterus or involves the parametrial tissues. Currently, this is an unusual event. Persistently elevated or rising serum β-hCG titers following evacuation of a mole may represent either an invasive mole or choriocarcinoma. Because tissue is generally not available to make the distinction, elevated serum β-hCG titers are usually attributed to persistent gestational trophoblastic disease without further classification. The risk of invasive mole progressing to choriocarcinoma appears to be no greater than that of progression from an intracavitary hydatidiform mole, and presumably, many lesions of invasive mole would regress spontaneously. Nonetheless, persistent or elevated β-hCG titers are treated with chemotherapy often in the absence of a histologic diagnosis, and the lesions nearly always respond.
EXAGGERATED PLACENTAL SITE
Exaggerated placental site is a benign, nonneoplastic lesion characterized by an increased number of implantation site intermediate trophoblastic cells that extensively infiltrate the endometrium and underlying myometrium. An exaggerated placental site may occur in association with normal pregnancy, an abortion, or a hydatidiform mole. An exaggerated placental site is composed of implantation site intermediate trophoblastic cells, which display an identical immunophenotypic profile to the intermediate trophoblastic cells found in the normal implantation site. It has been thought that this lesion represents an exaggeration of a normal physiologic process.
Morphologic Features
Exaggerated placental site is defined as an increased number of implantation site intermediate trophoblastic cells exceeding those normally present in the implantation site from an early pregnancy. The lesion is composed predominantly of mononucleate implantation site intermediate trophoblastic cells and variable numbers of multinucleated intermediate trophoblastic cells that extensively infiltrate the endomyometrium (Fig. 49.7). Despite the extensive infiltration by intermediate trophoblastic cells, the overall architecture of the implantation site is not disturbed. Endometrial glands may be completely surrounded by trophoblastic cells but are not destroyed, and similarly, the smooth muscle cells of the myometrium are separated by cords, nests, and individual implantation site intermediate trophoblastic cells that diffusely infiltrate the myometrium without producing necrosis (55). The surrounding decidua, however, may show degeneration and necrosis typical of a spontaneous abortion. Other features associated with gestation are usually present including hyalinized spiral arteries, hypersecretory glands, and chorionic villi. Exaggerated placental site is often associated with hydatidiform moles, especially complete moles. Despite the profuse infiltration of trophoblastic cells, the Ki-67 index of these cells is near zero (except those associated with complete hydatidiform moles), suggesting that the increased number of trophoblastic cells is not the result of de novo proliferation in the implantation site (18,55).
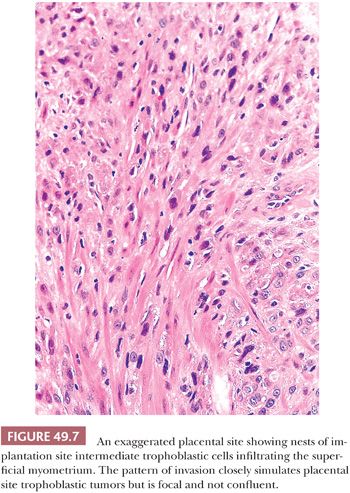
Differential Diagnosis
At times, exaggerated placental site may be difficult to distinguish from PSTT, particularly in curettings, as the diagnostic criteria for an exaggerated placental site are subjective (56). There are no reliable data quantifying the amount and extent of infiltration of implantation site intermediate trophoblast at different stages of normal gestation. Besides the overlap in the morphologic features of exaggerated placental site and PSTT, trophoblastic cells in early gestation have a primitive appearance and invade the uterine wall and spiral arteries extensively, thus invalidating the conventional histologic features used to distinguish a benign from a malignant process. However, exaggerated placental site is microscopic, lacks mitotic activity, is composed of intermediate trophoblastic cells separated by masses of hyaline, and usually is admixed with decidua and chorionic villi. In contrast, a lesion unaccompanied by villi and composed of confluent masses of implantation site intermediate trophoblastic cells that display unequivocal mitotic figures is classified as a PSTT. In addition, multinucleated trophoblastic cells are more often present in an exaggerated placental site than in a PSTT (55). Mitotic figures are useful in distinguishing a PSTT from an exaggerated placental site as the mitotic figures can only be detected in PSTTs. However, the “degenerated” appearance of intermediate trophoblastic cell nuclei makes it difficult to identify an unequivocal mitotic figure on hematoxylin and eosin (H&E) sections. In summary, the presence of chorionic villi (and/or fetal parts) and abundant multinucleated intermediate trophoblastic cells and the absence of confluence of intermediate trophoblastic cells in the endomyometrium suggest the diagnosis of an exaggerated placental site rather than a PSTT.
It has been reported that the Ki-67 nuclear labeling index using a Ki-67–specific (MIB-1) antibody is superior to the mitotic index as a diagnostic aid in the differential diagnosis of exaggerated placental site versus PSTT (18). Specifically, the Ki-67 index (mean ± standard deviation) of the trophoblastic cells in an exaggerated placental site is near zero in contrast to 14% ± 6.9% in a PSTT. Because Ki-67 labeling may occur in the lymphoid cells normally present at the placental site, it is important to be certain that Ki-67 labeling is assessed only in intermediate trophoblastic cells using strict cytologic criteria. In difficult cases, double immunostaining using an antibody against Ki-67 and intermediate trophoblastic markers including HSD3B1, HLA-G, and Mel-CAM (CD146) can assist in this distinction (4,7,57–59). The trophoblastic cells in an exaggerated placental site demonstrate an identical expression profile of trophoblast-associated markers to the intermediate trophoblastic cells in an early implantation site (Table 49.2). The application of immunohistochemistry in assisting the diagnosis of exaggerated placental site is described in the section “Immunohistochemistry Approach for Differential Diagnosis.”
Behavior and Treatment
An exaggerated placental site is a benign trophoblastic lesion that involutes following curettage. Exaggerated placental site is not associated with increased risk of persistent gestational trophoblastic disease. No specific treatment or follow-up is necessary. When an exaggerated placental site cannot be confidently distinguished from PSTT by morphology and immunohistochemistry, close follow-up with serial β-hCG titers is advisable.
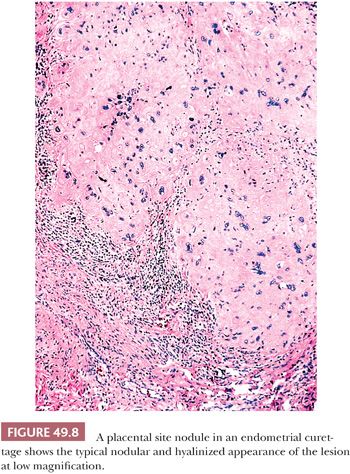
PLACENTAL SITE NODULE
A placental site nodule is a well-circumscribed hyalinized lesion composed of chorionic-type intermediate trophoblastic cells that are normally located in chorion laeve (fetal membrane) (Fig. 49.1). Typically, it is incidentally found in an endometrial biopsy, endocervical curetting, or cervical biopsy from a woman in the reproductive age group. Occasionally, placental site nodule can be found in fallopian tubes (60–64). In one study, 40% of placental site nodules were present in the endocervix, 56% in the endometrium, and 4% in the fallopian tube (6). A placental site nodule may be diagnosed several years after tubal ligation, suggesting either that it can be retained in the endometrium for extended periods of time or that the lesion develops as an unrecognized pregnancy. In this latter scenario, one must postulate that the tubes were incompletely transected or that they recanalized after surgery. It is generally thought that placental site nodule represents a retained noninvoluted placental site (65–67). However, morphologic and immunohistochemical studies have shown that placental site nodules are more closely related to intermediate trophoblastic cells from chorion laeve (fetal membrane) than from the placental site (6). It is also conceivable that the placental site nodule is an abnormal (i.e., blighted) gestation that never fully developed (6). This latter view is consistent with reports of these lesions as incidental findings in patients who never realized that they were pregnant.
Pathologic Features
The placental site nodule is a small, often microscopic finding (Fig. 49.8). When grossly visible, it presents as a yellow, tan, or hemorrhagic nodule measuring up to 1 cm in diameter and is located in the endometrium or superficial myometrium. On occasion, multiple nodules may be present. Microscopically, the placental site nodule has a discrete, well-circumscribed, lobulated border sometimes showing small irregular nests of cells projecting into the surrounding tissue. A thin layer of chronic inflammatory cells and decidual cells often encompasses the lesion. The chorionic-type intermediate trophoblastic cells within the placental site nodule are arranged singly, in nests and cords (55). They are embedded in abundant eosinophilic fibrillar extracellular matrix protein (6). The latter finding is a prominent feature of placental site nodules. The cells vary in size; many have relatively small uniform nuclei but others are large with mononucleate, irregular, and hyperchromatic nuclei (Fig. 49.9). Occasionally, scattered multinucleated cells are present. The cytoplasm of chorionic-type intermediate trophoblastic cells is abundant and eosinophilic to amphophilic, sometimes with conspicuous vacuolation. Morphologically and immunohistochemically, these cells resemble those in the chorion laeve and therefore they have been designated as chorionic-type intermediate trophoblastic cells. Gene expression of intermediate trophoblastic cells in the placental site nodules and in chorion laeve (fetal membrane) is the same. For example, they are only focally positive for hPL and CD146 (Mel-CAM) and are negative for mucin-4, in contrast to the intermediate trophoblastic cells in the implantation site, where these antigens are highly and diffusely expressed (6,68–70) (Fig. 49.2). Immunoreactivity for β-hCG is usually absent. In contrast to implantation site intermediate trophoblastic cell in the first trimester, the chorionic-type intermediate trophoblastic cells in placental site nodules are positive for p63 (70,71) (Fig. 49.2). Similar to the chorionic-type intermediate trophoblastic cells found in the fetal membranes of normal pregnancy, there is a low level of proliferation in the cells of placental site nodules as indicated by a few scattered Ki-67 labeled nuclei (6). In contrast, in the normal implantation site and even in exaggerated placental sites, the Ki-67 index of implantation site intermediate trophoblastic cells is zero (18).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


