Fig. 11.1
Germinoma. Large monomorphous tumor cells separated into lobules by thin fibrous septa.
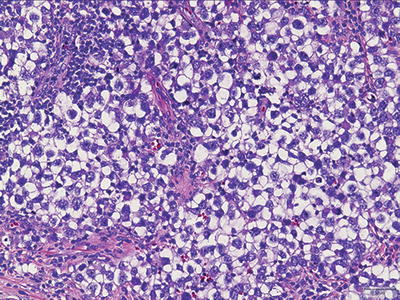
Fig. 11.2
Germinoma. Tumor cells have “squared off” nuclei, abundant clear cytoplasm, and distinct cell borders.
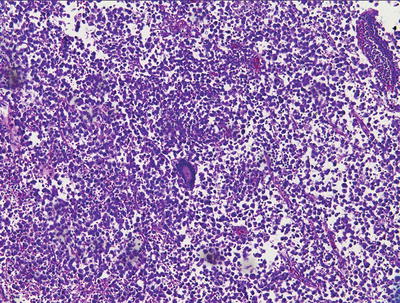
Fig. 11.3
Germinoma. Note the syncytiotrophoblastic giant cell.
Of practical significance is the fact that these tumors are not easily surgically accessible, often yielding very small samples. As such, in samples where the lymphocytic infiltrate predominates, the diagnostic tumor cells may be obscured and difficult to identify on hematoxylin and eosin-stained sections. Other samples may show an unusual single cell infiltration of the juxtaposed brain parenchyma similar to malignant gliomas or lymphomas. Such scenarios underscore the necessity of routinely employing immunohistochemical studies, including GCT markers, in the work-up of midline CNS lesions.
Yolk Sac Tumor
In contrast to germinomas, yolk sac tumors rarely occur in pure form. More commonly, they are a component of a mixed germ cell tumor. Cytologically, neoplastic cells are large and polygonal in shape with faint eosinophilic or clear cytoplasm and well-defined cytoplasmic borders. Nuclei are moderately atypical, but generally lack marked pleomorphism (Fig. 11.4). Tufts of malignant cuboidal-to-columnar tumor cells surrounding central blood vessels, known as Schiller-Duval bodies, are common though not universal, and not necessary for the diagnosis. The characteristic intercellular and extracellular PAS-positive/diastase-resistant eosinophilic hyaline globules and intercellular longitudinal bands of eosinophilic basement membrane material offer additional diagnostic clues.
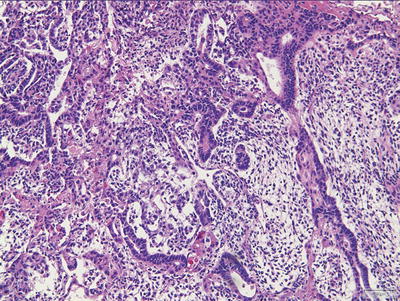

Fig. 11.4
Yolk sac tumor. Microcystic pattern.
Perhaps the most consistent finding in yolk sac tumors is the variety of different morphologic patterns often encountered within the same tumor; a mixture of patterns is the rule (Figs. 11.4 and 11.5). The most common among these is the reticular or microcystic pattern consisting of cysts lined by a loose network of flattened-to-cuboidal cells. A macrocystic pattern is seen when the microcysts coalesce. The polyvesicular vitelline pattern displays larger vesicles lined by flat-to-columnar epithelial cells. The endodermal sinus pattern shows a predominance of Schiller-Duval bodies and the papillary pattern shows papillae rimmed with tumor cells. Clusters of liver-like tumor cells are seen in the hepatoid pattern and neoplastic cells embedded in a matrix of basement membrane-rich material are evidence of parietal differentiation. Glandular, myxomatous, solid, and sarcomatoid patterns are often found in association with the aforementioned patterns.
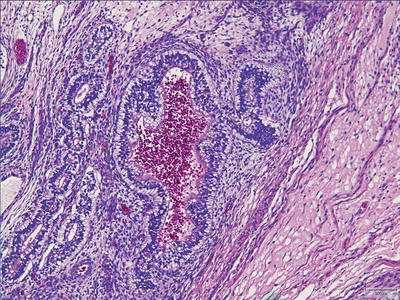

Fig. 11.5
Yolk sac tumor. Glandular pattern.
Embryonal Carcinoma
Tumor cells are highly atypical and are generally larger, more pleomorphic, and more carcinoma-like than those of germinoma (Fig. 11.6). Nuclei are oval-to-round and hyperchromatic with irregular nuclear contours and large single or multiple nucleoli. Their cytoplasm is fairly abundant, somewhat granular, and variably staining. Cytoplasmic borders are not well defined, accounting for the syncytial appearance of the tumor (Fig. 11.7). The malignant cells can be arranged in solid sheets, cords, papillae, or gland-like patterns. The so-called appliqué pattern, characterized by smudged, degenerate-appearing cells seen towards the periphery of tumor nests, imparts a superficial resemblance to choriocarcinoma. Necrosis is common and the mitotic rate is typically high. As with germinomas, syncytiotrophoblastic giant cells are not an infrequent finding. Small foci of neoplastic poorly differentiated stroma, considered by some investigators to be teratomatous in nature, may accompany embryonal carcinoma and account for chemotherapy failure.
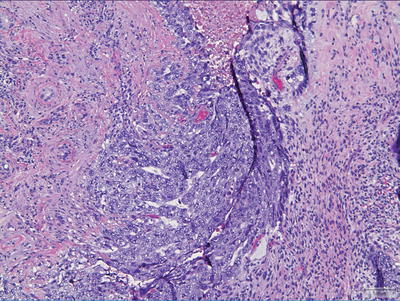
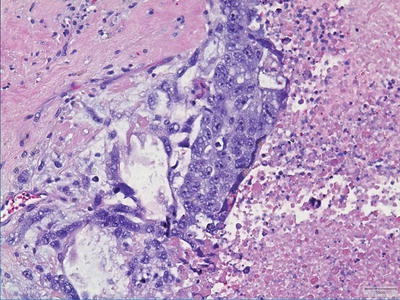

Fig. 11.6
Embryonal carcinoma. Tumor cells are larger than those of germinoma and more carcinoma-like.

Fig. 11.7
Embryonal carcinoma. Tumor cells show oval-to-round hyperchromatic nuclei with large nucleoli.
Teratoma
Akin to their gonadal counterparts, teratomas of the CNS can comprise both mature and immature elements. Pure mature teratomas tend to behave in an indolent fashion whereas teratomas occurring as part of a mixed GCT are more aggressive regardless of their degree of maturity. Their biologic behavior, thus, is likened to that of ovarian teratomas. Congenital teratomas, on the other hand, have a universally poor prognosis regardless of their composition.
Mature Teratoma
Mature teratomas are made up of an admixture of differentiated, adult-type tissues from more than one germ cell layer (Fig. 11.8). Skin and glial tissue are common ectodermal components and enteric, respiratory or transitional type tissues account for endodermal derivation. Focal increased cellularity, mitotic activity, and/or moderate cellular atypia are acceptable and should not prompt a diagnosis of immature teratoma, nor should the presence of “fetal” type tissues (e.g., fetal cartilage). The diagnosis of immaturity, rather, should only be made when tissues closely resemble embryonal (not fetal) type tissues (see below).
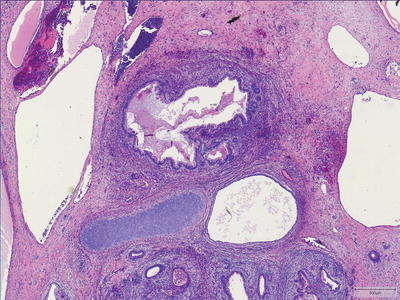

Fig. 11.8
Mature teratoma. Mature teratomas are made up of an admixture of differentiated tissues from more than one germ cell layer.
Immature Teratoma
Immature teratomas, by definition, contain varying amounts of incompletely differentiated tissues that resemble primitive embryonic tissues (Fig. 11.9). Most commonly, the immature tissue shows neural differentiation with rosette or tubule formation (i.e., primitive or embryonic-type neuroepithelium). Blastomatous-type stroma, consisting of small, round-to-spindled cells with hyperchromatic nuclei, apoptosis, and increased mitoses, is frequently encountered surrounding small, immature glands. Increased mitoses and apoptosis are not features of mature tissue, and can be helpful clues to the identification of immature elements. Any amount of immature component, no matter how small, is sufficient to render the diagnosis of immature teratoma. Following the convention of their ovarian counterparts, some observers choose to quantify the volume of the immature component and assign a grade (grade I–III) based on this volume. While the grading of immature teratomas in the ovary has documented prognostic significance, this has not been proven in the CNS to date. It is worth noting that maturation of a previously treated teratoma is a phenomenon often encountered secondary to effects of chemotherapy and irradiation. Conversely, malignant transformation of mature teratoma after treatment has also been documented [6].
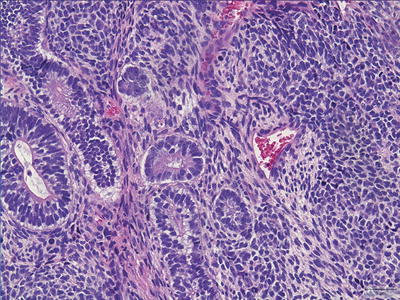

Fig. 11.9
Immature teratoma. Immature teratomas contain tissues that resemble primitive embryonic tissues. Note the immature neuroepithelium.
Congenital Teratoma
Congenital teratomas are the most common neonatal brain tumor and, by definition, occur within the first 60 days of life [7, 8]. Although, they are histologically similar to those occurring later in life, key differences exist. First and foremost is their location. In contrast to the infratentorial teratomas of older children, congenital teratomas are predominantly supratentorial. Due to their large size, however, a precise determination of their site of origin is often difficult [9]. Secondly, congenital teratomas are generally pure, either mature or immature. Finally, congenital teratomas carry a dismal prognosis (>90 % mortality rate) while those occurring in older children generally have a much better clinical outcome [8].
Choriocarcinoma
Choriocarcinomas are the most malignant GCTs but are, fortuitously, the least common among the primary tumors. They are extensively hemorrhagic and highly necrotic tumors comprising of two cell types: syncytiotrophoblasts and cytotrophoblasts (Fig. 11.10). Syncytiotrophoblasts are easily recognized as large multinucleated cells with smudged vesicular nuclei and dark eosinophilic-to-amphophilic cytoplasm. Cytotrophoblasts are more uniform and have single bland nuclei with vesicular chromatin and pale-to-amphophilic cytoplasm. Cytoplasmic borders are distinct. Syncytiotrophoblasts and cytotrophoblasts are arranged in a biphasic plexiform pattern similar to that seen in chorionic villi where sheets of cytotrophoblasts are surrounded (caped) by syncytiotrophoblasts. Mitoses are easily identifiable in the cytotrophoblastic component, but occur only exceptionally in syncytiotrophoblasts. Rarely, the cytotrophoblastic component dominates. This monomorphic pattern, which can be seen following chemotherapy, may be difficult to recognize and often requires immunohistochemical confirmation. As discussed earlier, it is important to recognize that scattered syncytiotrophoblasts are not uncommonly encountered in other GCTs and their presence alone is not diagnostic of choriocarcinoma.
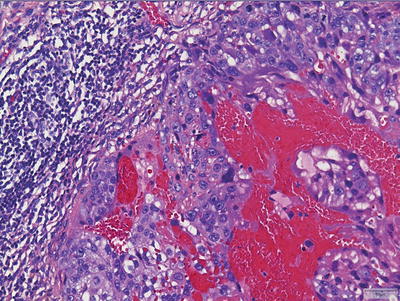

Fig. 11.10
Choriocarcinoma. Tumors show extensive hemorrhage and necrosis with recognizable syncytiotrophoblasts and cytotrophoblasts.
Mixed Germ Cell Tumors
As previously mentioned, mixed germ cell tumors comprise two or more of the previously described histologic variants. The relative percentage of each component should be reported. It is recommended that the tissue be, at minimum, extensively sampled if not entirely embedded to avoid underreporting of a GCT component.
Ancillary Immunohistochemical Studies
Routine use of ancillary immunohistochemical studies is standard of practice in the work-up of primary CNS GCTs. The most historically utilized immunostain in GCTs, placental alkaline phosphatase (PLAP), is now obsolete and has been replaced by superior, more specific and sensitive transcription markers, such as OCT4 [10]. OCT4 preferentially highlights germinomas and embryonal carcinomas and has the added advantage of being a nuclear marker allowing for easier interpretation (Fig. 11.11). CD30 shows strong membranous staining in embryonal carcinomas while other GCTs, including germinomas, are negative (Fig. 11.12). C-kit can also be exploited in the differential diagnosis of embryonal carcinoma versus germinoma as it shows strong and diffuse membranous staining in germinoma but focal or weak cytoplasmic staining in embryonal carcinomas [11–13].
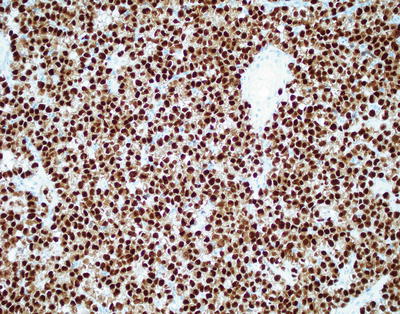
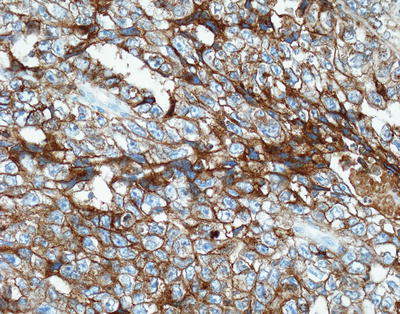

Fig. 11.11
Germinoma. OCT4 immunohistochemistry shows strong nuclear staining.

Fig. 11.12
Embryonal carcinoma. CD30 immunostain shows strong membranous staining.
SALL4 appears to be a fairly sensitive and specific pan-germ cell marker and can be used as a screening marker. A recent study by Mei et al. demonstrated 100 % sensitivity for germinomas, embryonal carcinomas, and yolk sac tumors (Fig. 11.13) [14]. Additionally, positive staining was observed in approximately two-thirds of teratomas and choriocarcinomas; all non-GCTs were negative for SALL4 staining [14].
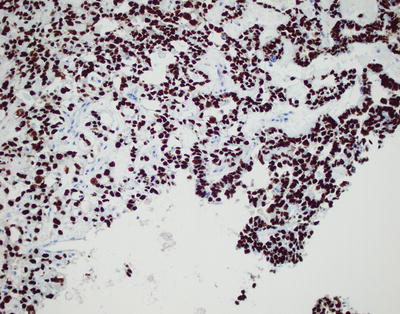

Fig. 11.13
Yolk sac tumor. SALL4, a pan-germ cell marker, shows strong nuclear staining.
Alpha-fetoprotein (AFP) has historically served as the marker of choice for yolk sac tumors. Staining, however, is often focal and patchy and generally varies among the different patterns of tumors. Additionally, abundant background staining is often observed [14]. Glypican-3 is touted as a more superior marker in diagnosing yolk sac tumors of the ovaries and testes. Glypican-3 offers more precise and easy to interpret staining characteristics as well as improved sensitivity (Fig. 11.14) [15]. Studies evaluating glypican-3 staining in CNS yolk sac tumors, however, are limited.
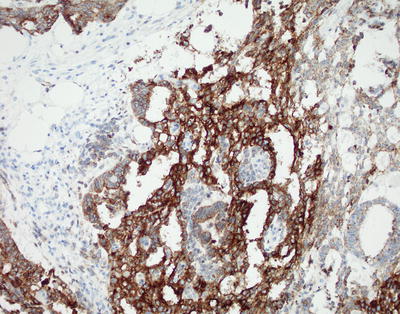

Fig. 11.14
Yolk sac tumor. Glypican 3 immunostain shows cytoplasmic staining.
βhCG and human placental lactogen (hPL) strongly stain the syncytiotrophoblastic cells of choriocarcinomas as well as those intermixed with other germ cell tumors [16, 17]. Cytotrophoblastic cells often are weakly positive or negative for these markers. Additionally, cytokeratins can also be used to highlight choriocarcinomas.
Histogenesis
GCTs are thought to arise from progenitor germ cells, mainly due to the facts that the germinoma component resembles progenitor cells very closely, intracranial GCTs resemble their extracranial counterparts morphologically and immunophenotypically, a single tumor can have multiple components (mixed GCTs) suggestive of differentiation of progenitor cells along various lines (embryonic and extraembryonic), and because none of the progenitor cells in the brain share any morphologic features with CNS GCTs. It is hypothesized that aberrant migration of the germ cell progenitors ventrally along the midline is responsible for the predominant midline location of these tumors throughout the body. Both testicular and CNS GCTs have been shown to have overexpression of wild type p53 and MDM2 proteins with a low incidence of TP53 gene mutation and a moderate incidence of MDM2 gene amplification pointing towards a common origin of these tumors [18]. Since p14ARF, a protein coded by the INK4a/ARF gene locus, functions as a tumor suppressor and regulates the interaction between the MDM2 and p53 proteins by stimulating degradation of MDM2, Iwato et al. further tested for gene mutations in the INK4a/ARF gene in 21 CNS GCTs. They found that 71 % of tumors (90 % of germinomas and 55 % of NGGCTs) had either a homozygous deletion (14/15) or a frameshift mutation (1/15) in this gene pointing towards a more central role for this protein in the development of CNS GCTs [19]. More evidence linking germ cell progenitors to CNS GCTs is the lack of methylation seen in gonadal and extragonadal GCTs, since the progenitor cells transiently lose methylation of imprinted genes during migration. Small nuclear ribonucleoprotein polypeptide N (SNRPN) is an imprinted gene with complete lack of methylation, which is common to GCTs and progenitor cells. However, Lee et al. showed that lack of methylation of SNRPN and other imprinted genes is also seen in neural stem cells in the brain providing an alternate hypothesis about the origin of CNS GCTs [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


