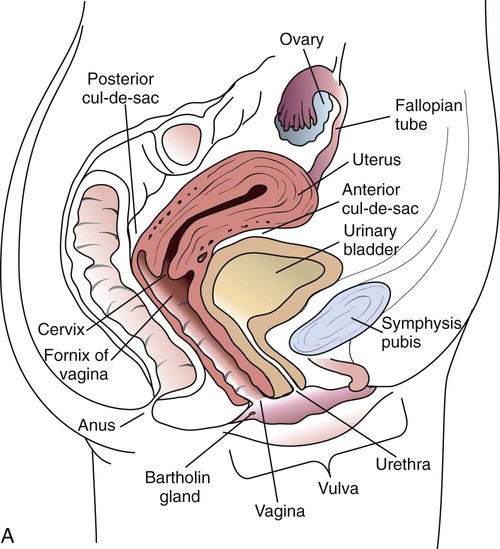
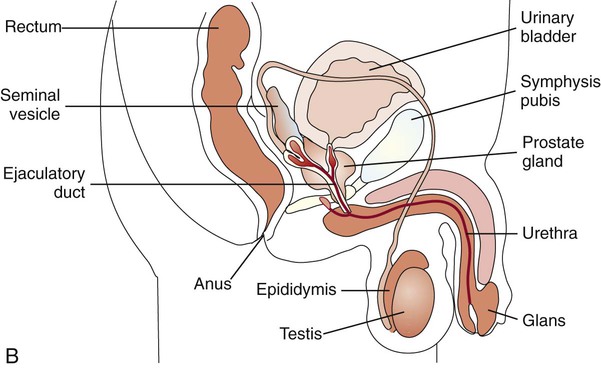
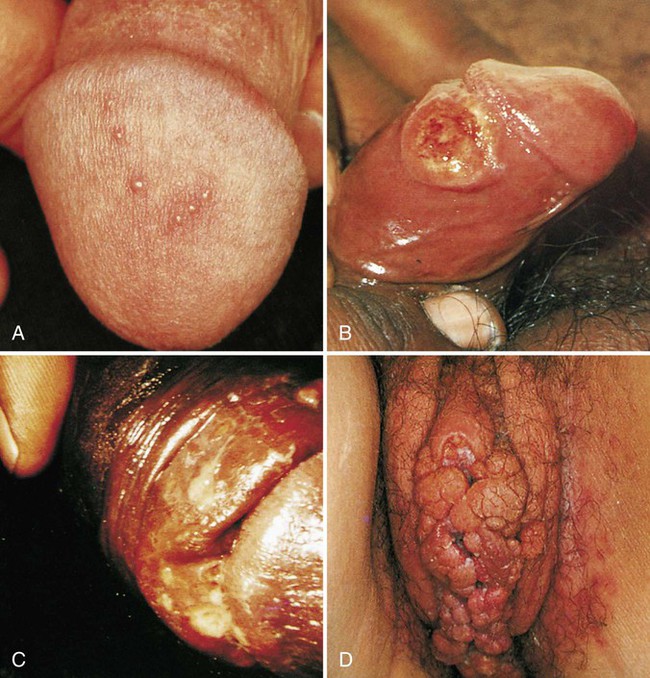
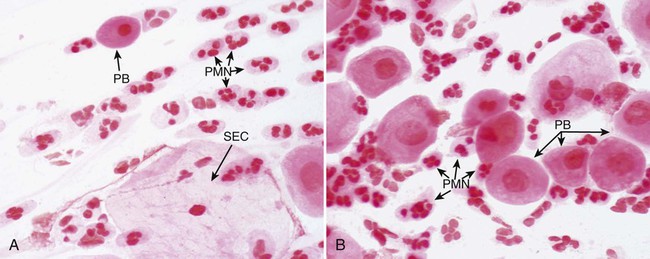
1. Describe the basic anatomy of the male and female reproductive systems. 2. Define the following conditions: vaginitis, cervicitis, proctitis, bartholinitis, pelvic inflammatory disease (PID), epididymitis, prostatitis, orchitis, neoplasia, urethritis, and dysuria. 3. List microorganisms that commonly are associated with vaginitis, cervicitis, and PID. 4. Describe the normal flora of the male and female genital tracts, and differentiate normal flora from pathogenic organisms. 5. List the media used to selectively isolate and differentiate genital tract pathogens including Modified Thayer-Martin (MTM), New York City (NYC), Colistin Nalidixic Agar (CNA), and JEMBEC System and the organisms capable of growth on each. 6. Compare and contrast the recommended use of various collection swabs for genital tract specimens including the organisms inhibited by each (cotton-tipped, with or without charcoal; calcium alginate; Dacron; rayon). 7. Determine specimen acceptability based on collection, transport, and diagnostic test orders for a genital tract specimen. 8. Explain the significance of gram-negative intracellular diplococci in genital specimens from both men and women. 9. Correlate signs and systems of infections with the results of laboratory diagnostic procedures for identification of the etiologic agent associated with infections of the genital tract. Familiarity with the anatomic structures is important for appropriate processing of specimens from genital tract sites and interpretation of microbiologic laboratory results. The key anatomic structures for the female and male genital tract in relation to other important structures are shown in Figure 74-1. The number of microorganisms that can cause genital tract infections is large. These organisms are diverse, representing all four major groups of microorganisms (bacteria, viruses, fungi, and parasites). The major causes of genital tract infections are listed in Table 74-1. TABLE 74-1 Major Causes of Genital Tract Infections and Sexually Transmitted Diseases Numerous organisms can cause genital lesions that are diverse in both their appearance and their associated symptoms (Figure 74-2). The agents and their features of infection are summarized in Table 74-2. Some of these infections, such as genital herpes (caused by HSV) or genital warts (caused by HPVs and discussed in Chapter 66), are common, whereas others, such as lymphogranuloma venereum and granuloma inguinale, are uncommon in the United States. Specific HPV genotypes infect mucosal cells in the cervix and can cause a progressive spectrum of abnormalities classified as low-grade and high-grade squamous intraepithelial neoplasia (process of rapid cell growth that is faster than normal and continues to grow, i.e., a tumor) and in some cases, progress to invasive cervical cancer. TABLE 74-2 Summary of Common Causes of Genital Lesions of the Skin and Mucous Membranes Although uncommon, there are other infectious causes of vaginitis. Three are briefly mentioned here because Gram stain of vaginal secretions may be helpful. First, Sobel described a number of premenopausal patients with a diffuse, exudative vaginitis with massive vaginal cell exfoliation, purulent vaginal discharge, and an occasional vaginal and cervical spotted rash. Laboratory findings included elevated pH of vaginal secretions. Also, numerous polymorphonuclear cells, an increased number of parabasal cells, the absence of gram-positive bacilli, and their replacement by occasional gram-positive cocci are observed on direct Gram stain (Figure 74-3). Basal cells appear as a result of the extensive exfoliation of epithelial cells. This clinical syndrome is referred to as desquamate inflammatory vaginitis. Symptoms associated with another disorder, lactobacillosis, resemble those of candidiasis and often follows antifungal therapy. Gram stain or wet mount typically reveals a large number of very long lactobacilli. These predominately anaerobic lactobacilli are 40 to 75 µ in length and are significantly longer than the average normal flora lactobacillus (5 to 15 µ). Finally, preexisting lesions due to other diseases may become secondarily infected with a mixed anaerobic flora of fusobacteria and spirochetes. This is referred to as fusiform-spirochete disease; this infection can progress rapidly. Gram stain examination reveals inflammatory cells in conjunction with gram-negative, fusiform bacterial morphotypes and spirochetes.
Genital Tract Infections
General Considerations
Anatomy
Genital Tract Infections
Sexually Transmitted Diseases and Other Lower Genital Tract Infections
Epidemiology/Etiologic Agents
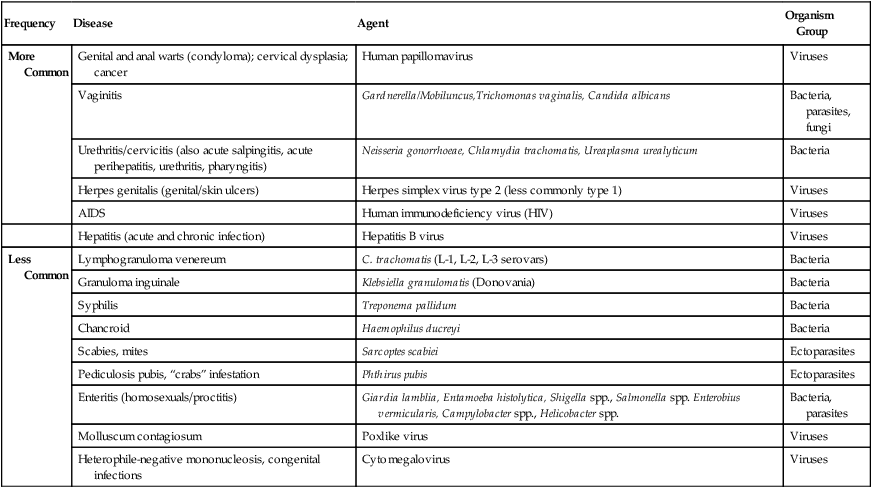
Frequency
Disease
Agent
Organism Group
More Common
Genital and anal warts (condyloma); cervical dysplasia; cancer
Human papillomavirus
Viruses
Vaginitis
Gardnerella/Mobiluncus,Trichomonas vaginalis, Candida albicans
Bacteria, parasites, fungi
Urethritis/cervicitis (also acute salpingitis, acute perihepatitis, urethritis, pharyngitis)
Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticum
Bacteria
Herpes genitalis (genital/skin ulcers)
Herpes simplex virus type 2 (less commonly type 1)
Viruses
AIDS
Human immunodeficiency virus (HIV)
Viruses
Hepatitis (acute and chronic infection)
Hepatitis B virus
Viruses
Less Common
Lymphogranuloma venereum
C. trachomatis (L-1, L-2, L-3 serovars)
Bacteria
Granuloma inguinale
Klebsiella granulomatis (Donovania)
Bacteria
Syphilis
Treponema pallidum
Bacteria
Chancroid
Haemophilus ducreyi
Bacteria
Scabies, mites
Sarcoptes scabiei
Ectoparasites
Pediculosis pubis, “crabs” infestation
Phthirus pubis
Ectoparasites
Enteritis (homosexuals/proctitis)
Giardia lamblia, Entamoeba histolytica, Shigella spp., Salmonella spp. Enterobius vermicularis, Campylobacter spp., Helicobacter spp.
Bacteria, parasites
Molluscum contagiosum
Poxlike virus
Viruses
Heterophile-negative mononucleosis, congenital infections
Cytomegalovirus
Viruses
Clinical Manifestations
Lesions of the Skin and Mucous Membranes.
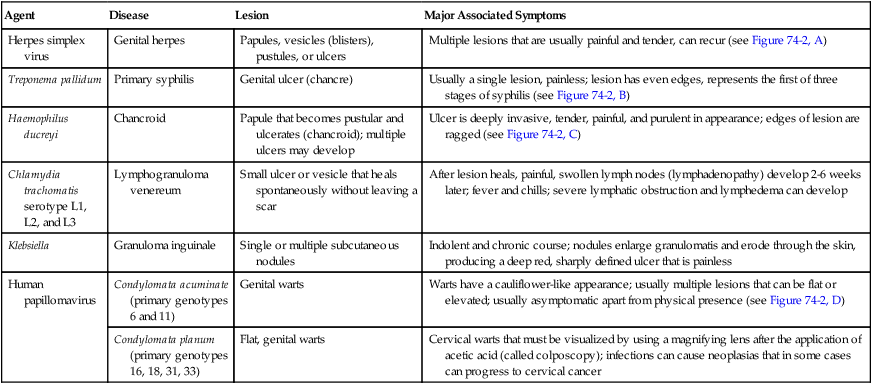
Agent
Disease
Lesion
Major Associated Symptoms
Herpes simplex virus
Genital herpes
Papules, vesicles (blisters), pustules, or ulcers
Multiple lesions that are usually painful and tender, can recur (see Figure 74-2, A)
Treponema pallidum
Primary syphilis
Genital ulcer (chancre)
Usually a single lesion, painless; lesion has even edges, represents the first of three stages of syphilis (see Figure 74-2, B)
Haemophilus ducreyi
Chancroid
Papule that becomes pustular and ulcerates (chancroid); multiple ulcers may develop
Ulcer is deeply invasive, tender, painful, and purulent in appearance; edges of lesion are ragged (see Figure 74-2, C)
Chlamydia trachomatis serotype L1, L2, and L3
Lymphogranuloma venereum
Small ulcer or vesicle that heals spontaneously without leaving a scar
After lesion heals, painful, swollen lymph nodes (lymphadenopathy) develop 2-6 weeks later; fever and chills; severe lymphatic obstruction and lymphedema can develop
Klebsiella
Granuloma inguinale
Single or multiple subcutaneous nodules
Indolent and chronic course; nodules enlarge granulomatis and erode through the skin, producing a deep red, sharply defined ulcer that is painless
Human papillomavirus
Condylomata acuminate (primary genotypes 6 and 11)
Genital warts
Warts have a cauliflower-like appearance; usually multiple lesions that can be flat or elevated; usually asymptomatic apart from physical presence (see Figure 74-2, D)
Condylomata planum (primary genotypes 16, 18, 31, 33)
Flat, genital warts
Cervical warts that must be visualized by using a magnifying lens after the application of acetic acid (called colposcopy); infections can cause neoplasias that in some cases can progress to cervical cancer
Vaginitis.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Genital Tract Infections