General Concepts, Germ Cell Tumors
Steven S. Shen, MD, PhD
Jae Y. Ro, MD, PhD
TERMINOLOGY
Synonyms
Germ cell tumor (GCT), seminoma, nonseminomatous germ cell tumor (NSGCT), mixed germ cell tumor (MGCT)
Definitions
Diverse group of tumors arising from totipotential germ cells with embryonic or extraembryonic differentiation
EPIDEMIOLOGY
Age Range
Most GCTs occur between 20-50 years of age with peak incidence at 30 years
Seminoma occurs at age ranging 35-45 years
NSGCTs occur at age ranging 25-35 years (10 years younger than seminoma)
Ethnicity Relationship
Incidence is higher in Western and Northern Europe, Australia/New Zealand, and North America (5.4-7.9 per 100,000)
Incidence is lower in Africa, Caribbean, and Asia (2 per 100,000)
Incidence
Estimated 8,400 new cases and 380 deaths from testicular cancer in USA in 2009 (American Cancer Society)
Approximately 49,000 new cases and 9,000 deaths each year worldwide (2002 data)
Worldwide incidence has more than doubled in last 40 years
Natural History
Intratubular germ cell neoplasia (ITGCN) is a precursor to most GCTs except for spermatocytic seminoma and infantile germ cell tumors
NSGCTs are more likely than seminoma to present with metastasis
Choriocarcinoma often presents with early vascular dissemination to lung, liver, and bone
May present with choriocarcinoma syndrome (hemorrhagic metastasis)
Metastasis of GCTs occurs in stepwise pattern of lymphatic spread through testicular mediastinum to retroperitoneal lymph nodes
ETIOLOGY/PATHOGENESIS
Cytogenetic Changes
GCTs arising in prepubertal gonads (teratoma and yolk sac tumor) are usually diploid
GCTs in postpubertal men typically have 1 or more copies of chromosome 12p (most commonly i[12p]), and other forms of 12p abnormalities and aneuploidy
Approximately 80% of GCTs have at least 1 isochromosome 12 (i[12p])
Other genetic changes in postpubertal men include loss of chromosome 11, 13, 18, and Y, and gains of 7, 8, and X
Spermatocytic seminoma may be either diploid or aneuploid and may show loss of chromosome 9
Risk Factors
Prior history of GCT
Positive family history of GCT
Cryptorchidism
Testicular dysgenesis
Klinefelter syndrome
Infertility
CLINICAL IMPLICATIONS
Clinical Presentation
Often unilateral painless testicular swelling or mass (bilaterality is rare: < 2%)
Gynecomastia or exophthalmos may be presenting symptom (related to human chorionic gonadotropin [hCG] production)
Approximately 10% may present with symptoms related to metastasis at initial presentation
Elevation of serum tumor markers, including lactate dehydrogenase(LDH), α-fetoprotein(AFP), hCG
Laboratory Tests
Elevated serum AFP usually seen in yolk sac tumor
Highly elevated serum hCG suggests choriocarcinoma; borderline hCG elevation is not uncommon in seminoma and in germ cell tumors with syncytiotrophoblasts
Serum levels of LDH, AFP, and hCG are incorporated into TNM staging
Treatment
Treatment options depend on TNM stage and whether tumor is seminoma or NSGCT
Stage I seminoma
Radical inguinal orchiectomy followed by surveillance protocol (serum markers, chest radiographs, and CT scan), single-dose carboplatin adjuvant therapy, or radiation therapy
Stage I NSGCT
Radical inguinal orchiectomy followed by retroperitoneal lymph node dissection (RPLND), surveillance protocol, or cisplatin-based adjuvant chemotherapy
Stage II seminoma
Radical inguinal orchiectomy followed by radiation or cisplatin-based adjuvant therapy
Stage II NSGCT
Radical inguinal orchiectomy followed by RPLND, RPLND and chemotherapy, or chemotherapy and delayed RPLND
Stage III seminoma or NSGCT
Radical inguinal orchiectomy followed by multidrug chemotherapy
Prognosis
Depends on histologic type, stage, and treatment
Most types have favorable prognosis and respond well to chemotherapy &/or radiation therapy, as appropriate for tumor type
Overall 95% survival rate in USA
Morphologic prognostic factors
Lymphovascular invasion (pathologic stage at least pT2)
Proportion of embryonal carcinoma (> 80% poor prognosis)
Proportion of teratoma component (> 50% favorable prognosis)
Others: Tumor size (> 4 cm) and rete testis invasion (for seminoma)
Imaging Findings
General Features
Testicular ultrasound may detect a testicular mass
Abdominopelvic computed tomographic (CT) scan may detect retroperitoneal lymph node metastasis
Chest radiograph and CT scan may detect lung metastasis
Magnetic resonance imaging (MR) may detect metastasis to bone and brain
MACROSCOPIC FINDINGS
Anatomic Features
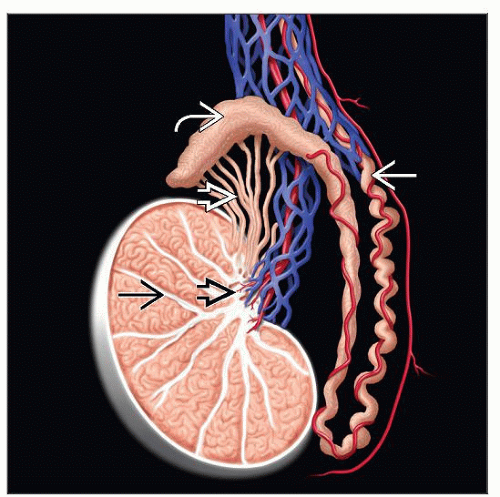
Testes are paired ovoid organ with average weight of 15-19 g and dimension of 2 x 3 x 4 cm
Surrounded by thick capsule composed of 3 layers: Tunica vaginalis (outer layer), albuginea (middle), and vasculosa (inner)
Posterior mediastinum contains blood vessels, lymphatics, and rete testis
Fibrous septae divide testis into approximately 250 lobules: Seminiferous tubules and interstitium
GCTs replace normal structures and present with tumoral pattern
General Features
Testicular mass with variable appearance depending on histologic type and component
Specimen Handling
Radical Resection
Orient specimen and ink appropriately, if necessary
Procure spermatic cord margin before specimen is opened/bivalved
Submit tumor entirely if small (< 2 cm)
For large tumors, at least 1 section per cm tumor
Section to include areas with different appearance
Section to include hemorrhagic and necrotic area (usually high-grade component, such as embryonal or choriocarcinoma)
More sections may be required in pure seminoma to rule out other germ cell components, especially if there are areas of hemorrhage and necrosis or serum AFP levels are elevated
Section to include rete testis and epididymis
Section to include uninvolved testicular parenchyma adjacent to tumor
At least 1 section of uninvolved testicular parenchyma away from tumor
Subtotal Resection
Orient specimen and ink resection margins appropriately
Perpendicular section of tumor with margin for possible frozen section for margin and diagnosis
Sections usually include entire tumor
Section to include uninvolved testicular parenchyma
MICROSCOPIC FINDINGS
Normal Anatomy and Histology
Histologic compartment of testis
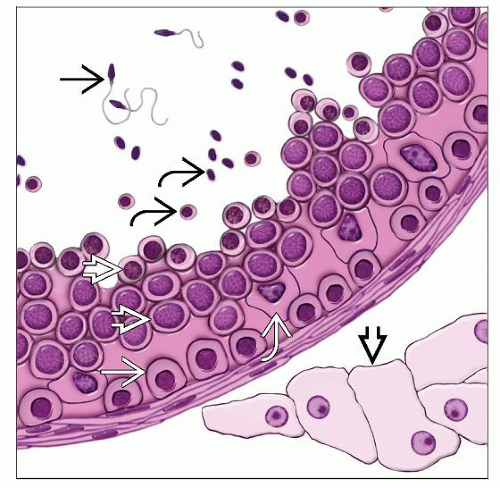
Testis is composed of seminiferous tubules and interstitium
Seminiferous tubules and spermatogenesis
Composed of Sertoli cells and germ cells in varying stages of differentiation or maturation
Germ cells mature from base to center of lumen and are divided into different stages based on their levels of maturation
Spermatogonia: Situated adjacent to basement membrane; small, round, dense nuclei with finely granular and vesicular chromatin and small nucleolus, clear or basophilic cytoplasm
Primary spermatocytes: More centrally located; largest cell type; variable nuclear appearance, clumped chromatin (spireme type), beaded cytoplasm
Secondary spermatocytes: More centrally located; smaller and fewer than primary spermatocytes; coarsely granular chromatin; no nucleoli
Spermatids: Located near lumen; small cells with darkly stained chromatin
Spermatozoa: Located in lumen; elongated eccentric nucleus with long cytoplasmic tail
Sertoli cells: Elongated pyramidal cells attached to basal lamina (10-12 Sertoli cells/tubules; germ cells:Sertoli cells ratio ~ 13:1)
Interstitium is divided into intertubular and peritubular regions
Peritubular region contains basement membrane and thin lamina propria
Intertubular interstitium contains blood vessels, lymphatics, nerve, and Leydig cells
General Features
World Health Organization (WHO) Histologic Classification of Testicular Germ Cell Tumors
Germ cell tumors
Intratubular germ cell neoplasia (ITGCN), unclassified
Other types
Tumors of 1 histologic type (pure forms)
Seminoma
Seminoma with syncytiotrophoblastic cells
Spermatocytic seminoma (SS)
Embryonal carcinoma (EC)
Yolk sac tumor (YST)
Trophoblastic tumors: Choriocarcinoma, monophasic choriocarcinoma, placental site trophoblastic tumor
Teratoma: Dermoid cyst, monodermal teratoma (carcinoid tumor), teratoma with somatic-type malignancies
Tumors of more than 1 histologic type (mixed forms; mixed NSGCT)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree