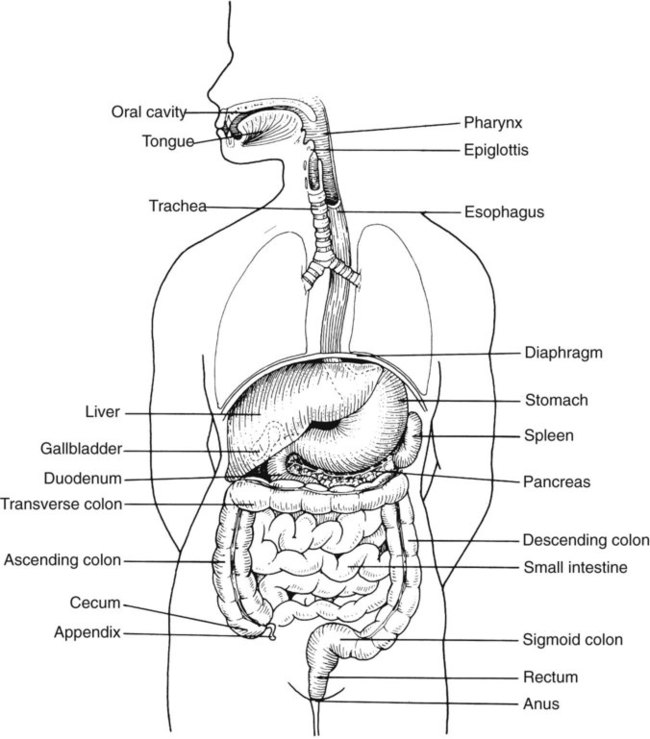
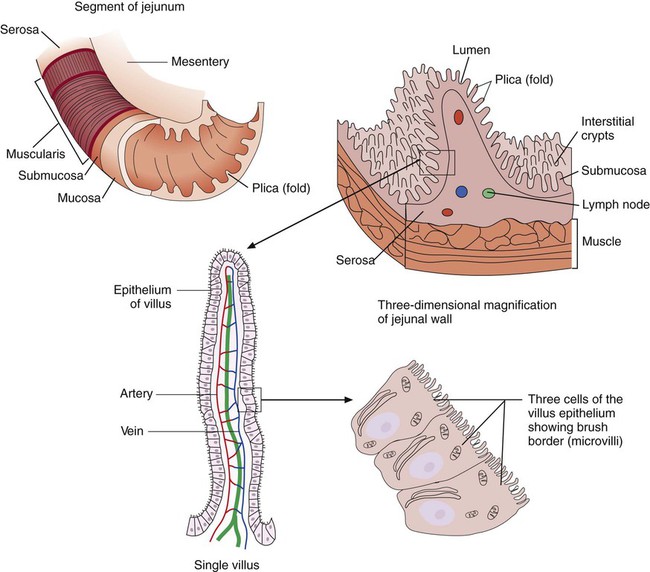
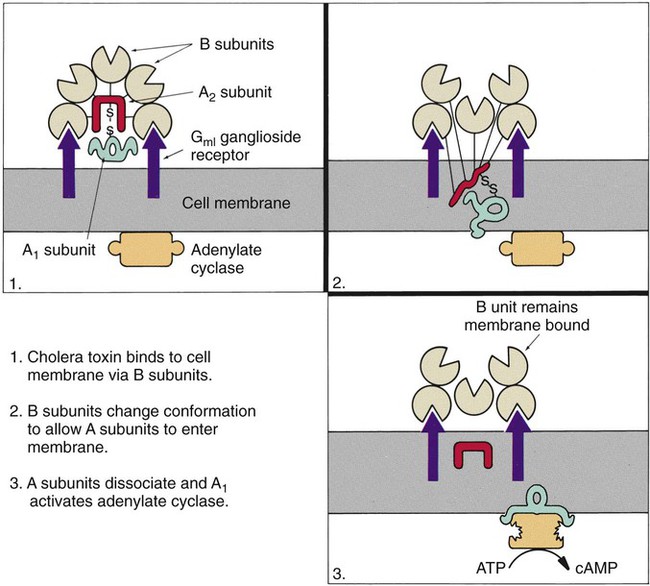
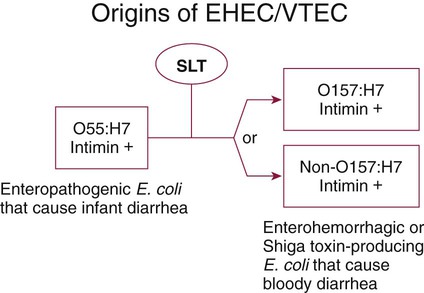
Chapter 75 1. Describe the general anatomy of the gastrointestinal tract and the relationship to transmission of infectious disease. 2. Differentiate normal flora from pathogenic organisms, and describe the relative numbers of organisms distributed throughout the gastrointestinal tract. 3. Identify nonbacterial agents of infection of the gastrointestinal tract, and name their associated diseases. 4. Describe the innate immunity as it relates to the gastrointestinal tract, including physical, chemical, and bacterial components. 5. Differentiate infections of the upper and lower gastrointestinal tract based on clinical manifestations including watery diarrhea and bloody diarrhea (dysentery). 6. Identify the major cause for antimicrobial therapy–associated diarrhea and the proper laboratory diagnostic procedure for identification, including the toxin assay. 7. Identify the most common causes for watery diarrhea, dysentery, pseudomembranous colitis, and infant botulism. 8. Describe the bacterial pathogenic mechanisms associated with gastrointestinal disease, including the presence and function of enterotoxins, attachment, and invasion mechanisms. 9. Determine the adequacy of a specimen based on collection, transport, and specimen type for the diagnosis of gastrointestinal infections. 10. Define the following media, including the organisms identified and the chemical properties associated with the selection and differentiation within the media (MAC, SMAC, EMB, HEK, XLD, SS, and Campy). 11. List the organisms and microbial products that can be detected by non-culture methods. 12. Correlate patient signs and symptoms with laboratory results for the identification of the gastrointestinal pathogen. We are all connected to the external environment through our gastrointestinal (GI) tract (Figure 75-1). What we swallow enters the GI tract and passes through the esophagus into the stomach, through the small and large intestines, and finally to the anus. During passage, fluids and other components are added to this material as secretory products of individual cells and as enzymatic secretions of glands and organs, and they are removed from this material by absorption through the gut epithelium. The major components of the tract are listed in Box 75-1. The nature of the epithelial cells lining the GI tract varies with each portion. The lining of the GI tract is called the mucosa. Because of the differing nature of the mucosal surfaces of various segments of the bowel, specific infectious disease processes tend to occur in each segment. The wall of the small intestine has folds that have millions of tiny, hairlike projections called villi. Each villus contains an arteriole, venule, and lymph vessel (Figure 75-2). The function of villi is to absorb fluids and nutrients from the intestinal contents. Epithelial cells lining the surface of villi have a surface resembling a fine brush, referred to as a brush border. The brush border is formed by nearly 2000 microvilli per epithelial cell. Intestinal digestive enzymes are produced in brush border cells toward the top of the villi. Villi and microvilli help make the small intestine the primary site of digestion and absorption by significantly increasing the surface area; more than 90% of physiologic net fluid absorption occurs here. Mucus-secreting goblet cells are found in large numbers of villi and intestinal crypts. Similar to the small intestine, the large intestine is composed of several segments (see Box 75-1). The wall of the large intestine consists of columnar epithelial cells, many of which are mucus-producing goblet cells. In contrast to the small intestine, there are no villous projections into the lumen. The remaining excess fluid within the GI tract is resorbed through the cells lining the large intestine before waste is finally discharged through the rectum. In addition to the previously discussed components of the GI tract, numerous other organs and structures are either located in the main digestive organs or open into them. These accessory organs and structures include the salivary glands, tongue, teeth, liver, gallbladder, and pancreas. Except for the teeth and salivary glands, these organs are illustrated in Figure 75-1. • By changing the delicate balance of water and electrolytes in the small bowel, resulting in massive fluid secretion. In many cases, this process is mediated by enterotoxin production. This is a noninflammatory process. • By causing cell destruction or a marked inflammatory response following invasion of host cells and possible cytotoxin production, usually in the colon. • By penetrating the intestinal mucosa, with subsequent spread and multiplication in lymphatic or reticuloendothelial cells outside of the bowel; these infections are considered systemic infections. Examples of microorganisms for each of these pathogenic mechanisms are listed in Table 75-1. TABLE 75-1 Examples of Microorganisms That Cause GI Infection for Each Primary Pathogenic Mechanism The classic example of an enterotoxin is that of Vibrio cholerae (Figure 75-3). This toxin consists of two subunits, A and B. The A subunit is composed of one molecule of A1, the toxic moiety, and one molecule of A2, which binds an A1 subunit to five B subunits. The B subunits bind the toxin to a receptor (a ganglioside, an acidic glycolipid) on the intestinal cell membrane. Once bound, the toxin acts on adenylate cyclase enzyme, which catalyzes the transformation of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Increased levels of cAMP stimulate the cell to actively secrete ions into the intestinal lumen. To maintain osmotic stabilization, the cells then secrete fluid into the lumen. The fluid is drawn from the intravascular fluid store of the body. Patients therefore can become dehydrated and hypotensive rapidly. V. cholerae inhabits sea and stagnant water and is spread in contaminated water. The organisms have been isolated from coastal waters of several states, and sporadic cases of cholera occur in the United States. Additional information about V. cholerae is provided in Chapter 26. E. coli strains seem to possess virulence mechanisms of many types. Some strains produce a cytotoxin capable of destroying epithelial cells and blood cells. Certain strains produce a cytotoxin that affects Vero cells (African green monkey kidney cells) and resemble the cytotoxin produced by Shigella dysenteriae (Shiga toxin); such strains of E. coli are associated with hemorrhagic colitis and the sequelae following infection of hemolytic-uremic syndrome (HUS) and thrombotic thrombocytopenia purpura (TTP). These strains of E. coli are referred to as enterohemorrhagic E. coli (EHEC), also referred to as serotoxigenic or STET/VTEC. See Chapter 20 for more information related to toxigenic E. coli. Table 75-2 summarizes the key pathogenic features of the primary groups of diarrheogenic E. coli. TABLE 75-2 Overview of the Primary Groups of E. coli That Cause Diarrhea in Humans An organism’s ability to cause disease can also depend on its ability to colonize and adhere to the bowel. To illustrate, ETEC must be able to adhere to and colonize the small intestine, as well as produce an enterotoxin. These organisms produce an adherence antigen, called colonization factor antigen (CFA). Certain strains of E. coli referred to as the enteropathogenic E. coli (EPEC) attach and then adhere to the intestinal brush border. This localized adherence is mediated by the production of pili. Subsequent to attaching, EPEC disrupts normal cell function by effacing the brush epithelium, thereby causing diarrheal disease. This complete process is referred to as attachment and effacement. Genes responsible for the initial adherence of ETEC, EHEC, and EPEC to intestinal epithelial cells reside on a transmissible plasmid. EHEC has the same ability to attach to intestinal epithelial cells and cause effacement. In addition, EHEC produces a Shiga toxin that spreads to the bloodstream, causing systemic damage to vascular endothelial cells of various organs, including kidney, colon, small intestine, and lung. EHEC is believed to have arisen as a result of an EPEC strain having become infected with a bacteriophage carrying the Shiga toxin gene (Figure 75-4).
Gastrointestinal Tract Infections
Anatomy
Gastroenteritis
Pathogenesis
Microbial Factors
Primary Pathogenic Mechanisms.
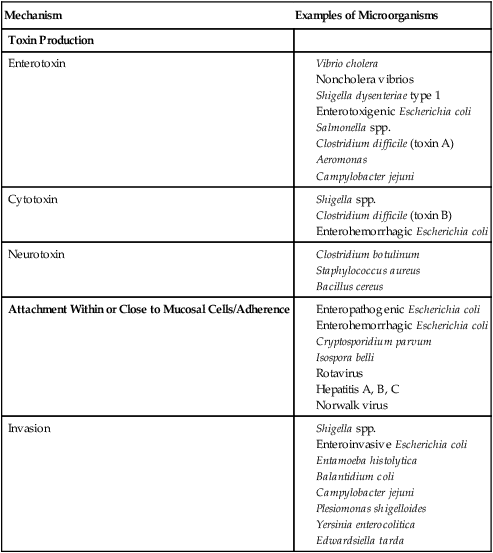
Mechanism
Examples of Microorganisms
Toxin Production
Enterotoxin
Cytotoxin
Neurotoxin
Attachment Within or Close to Mucosal Cells/Adherence
Invasion
Toxins
Type
Primary Mode of Pathogenesis
Other Comments
Enterotoxigenic (ETEC)
Produces heat-labile (LT) or heat stable (ST) enterotoxins; genes of both toxins reside on a plasmid; LTs are closely related in structure and function to cholera toxin; STs result in net intestinal fluid secretion by stimulating guanylate cyclase
Common cause of traveler’s diarrhea; infects all ages
Enteroaggregative (EAEC)
Binds to small intestine cells via fimbriae encoded by a large molecular weight plasmid, forming small clumps of bacteria on the cell surface; other plasmid-borne virulence factors include structured pilin, a heat-stable enterotoxin, novel anti-aggregative protein, and a heat-labile enterotoxin, all believed to be the cause of the associated diarrhea
Infects primarily young children
Enteroinvasive (EIEC)
Pathogenesis has yet to be totally elucidated; studies suggest that mechanisms by which diarrhea results are virtually identical to those of Shigella spp.
Very difficult to distinguish from Shigella spp. and other E. coli strains
Enteropathogenic (EPEC)
Initially attaches in the colon and small intestine and then becomes intimately adhered to intestinal epithelial cells, subsequently causing the loss of enterocyte microvilli (effacement); genes for attachment/effacement reside in a cluster on the bacterial chromosome (i.e., pathogenicity island)
Diarrhea in infants, particularly in large urban hospitals
Enterohemorrhagic (EHEC) OR
Attaches to and effaces gut epithelial cells in a similar manner as EPEC; in addition, EHEC elaborates shiga toxins
Although many outbreaks are caused by E. coli O157:H7, other serotypes have been implicated in outbreaks and sporadic cases
Gene recombination among strains makes classification difficult
Enterohemorrhagic (EHEC); or serotoxigenic (STEC); verotoxigenic (VTEC) (newest, terminology)
Produce one or more shiga toxins referred to as verocytotoxins. Attaches to and effaces gut epithelial cells in a similar manner as EPEC
0157 STEC serotypes; contains most common serotypes 0157 : H7 and nonmotile 0157 : NM. There are more than 150 non-0157 serotypes that have been isolated from patients with diarrhea or hemolytic uremic syndrome
Attachment.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree