Gastrointestinal Motility and Water Flux; Emesis; Biliary and Pancreatic Disease
GASTROINTESTINAL MOTILITY
The gastrointestinal (GI) tract is in a continuous contractile, absorptive, and secretory state. The control of this state is complex, with contributions by the muscle and epithelium, local nerves of the enteric nervous system (ENS), the autonomic nervous system (ANS), and circulating hormones. Of these, perhaps the most important regulator of physiological gut function is the ENS (Figure 46–1).
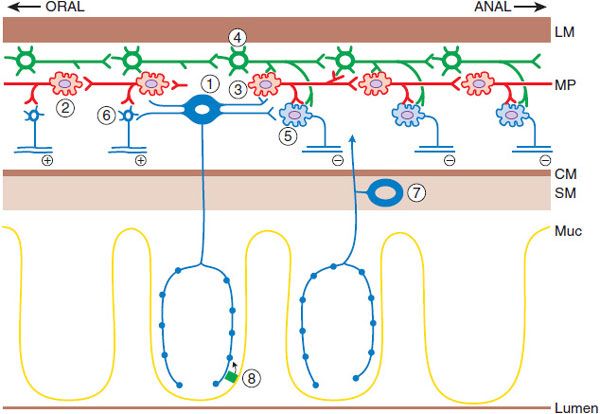
Figure 46–1 The neuronal network that initiates and generates the peristaltic response. Mucosal stimulation leads to release of serotonin by enterochromaffin cells (8), which excites the intrinsic primary afferent neurons (1), which then communicate with ascending (2) and descending (3) interneurons in the local reflex pathways. The reflex results in contraction at the oral end via the excitatory motor neuron (6) and aboral relaxation via the inhibitory motor neuron (5). The migratory myoelectric complex (see text) is shown here as being conducted by a different chain of interneurons (4). Another intrinsic primary afferent neuron with its cell body in the submucosa also is shown (7). MP, myenteric plexus; CM, circular muscle; LM, longitudinal muscle; SM, submucosa; Muc, mucosa. (Adapted with permission of Annual Reviews, from Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol, 1999;61:117–142. Permission conveyed through Copyright Clearance Center, Inc.)
The ENS is an extensive collection of nerves that constitutes the third division of the ANS. It is the only part of the ANS truly capable of autonomous function if separated from the central nervous system (CNS). The ENS lies within the wall of the GI tract organized into 2 connected networks of neurons and nerve fibers: the myenteric (Auerbach) plexus, found between the circular and longitudinal muscle layers, and the submucosal (Meissner) plexus, located in the submucosa. The former is largely responsible for motor control, whereas the latter regulates secretion, fluid transport, and blood flow. The ENS and the ANS are also involved in host defense and innervate organs and cells of the immune system.
GENERATION AND REGULATION OF GI ACTIVITY
The ENS is responsible for the largely autonomous nature of most GI activity. This activity is organized into relatively distinct programs that respond to input from the local environment of the gut, as well as the ANS-CNS. Each program consists of a series of complex, but coordinated, patterns of secretion and movement that show regional and temporal variation. The fasting program of the gut is called the MMC (migrating myoelectric complex when referring to electrical activity and migrating motor complex when referring to the accompanying contractions) and consists of a series of 4 phasic activities. The most characteristic, phase III, consists of clusters of rhythmic contractions that occupy short segments of the intestine for a period of 6-10 min before proceeding caudally (toward the anus). Phase II of the MMC is associated with the release of the peptide hormone motilin. Motilin agonists stimulate motility in the proximal gut. One whole MMC cycle (i.e., all 4 phases) takes ~80-110 min. The MMC occurs in the fasting state, helping to sweep debris caudad in the gut and limiting the overgrowth of luminal bacteria. The MMC is interrupted by the fed program in intermittently feeding animals such as humans. The fed program consists of high-frequency (12-15/min) contractions that are either propagated for short segments (propulsive) or are irregular and not propagated (mixing).
Peristalsis is a series of reflex responses to a bolus in the lumen of a given segment of the intestine; the ascending excitatory reflex results in contraction of the circular muscle on the oral side of the bolus, whereas the descending inhibitory reflex results in relaxation on the anal side. The net pressure gradient moves the bolus caudad. Motor neurons receive input from ascending and descending interneurons (which constitute the relay and programming systems) that are of 2 broad types, excitatory and inhibitory. The primary neurotransmitter of the excitatory motor neurons is acetylcholine (ACh). The principal neurotransmitter in the inhibitory motor neurons appears to be NO, although important contributions may also be made by ATP, vasoactive intestinal peptide (VIP), and pituitary adenylyl cyclase–activating peptide (PACAP). Enterochromaffin cells, scattered throughout the epithelium of the intestine, release serotonin (5HT) to initiate many gut reflexes by acting locally on enteric neurons. Excessive release of 5HT from the gut wall (e.g., by chemotherapeutic agents) leads to vomiting by actions of 5HT on vagal nerve endings in the proximal small intestine. Compounds targeting the 5HT system are important modulators of motility, secretion, and emesis.
Other cell types are important, including the interstitial cells of Cajal, distributed within the gut wall and responsible for setting the electrical rhythm and the pace of contractions in various regions of the gut. These cells also translate or modulate excitatory and inhibitory neuronal communication to the smooth muscle.
EXCITATION-CONTRACTION COUPLING IN GI SMOOTH MUSCLE
Control of tension in GI smooth muscle is dependent on the intracellular Ca2+ concentration. There are basically 2 types of excitation-contraction coupling in these cells. Ionotropic receptors can mediate changes in membrane potential, which in turn activate voltage-dependent Ca2+ channels to trigger an influx of Ca2+ (electromechanical coupling); metabotropic receptors activate various signal transduction pathways to release Ca2+ from intracellular stores (pharmaco-mechanical coupling). Inhibitory receptors act via PKA and PKG and lead to hyperpolarization, decreased cytosolic [Ca2+], and reduced interaction of actin and myosin. As an example, NO may induce relaxation via activation of guanylyl cyclase-cyclic GMP pathway and cause the opening of several types of K+ channels.
FUNCTIONAL AND MOTILITY DISORDERS OF THE BOWEL
GI motility disorders are a heterogeneous group of syndromes. Typical motility disorders include achalasia of the esophagus (impaired relaxation of the lower esophageal sphincter associated with defective esophageal peristalsis that results in dysphagia and regurgitation), gastroparesis (delayed gastric emptying), myopathic and neuropathic forms of intestinal dysmotility, and others. These disorders can be congenital, idiopathic, or secondary to systemic diseases (e.g., diabetes mellitus or scleroderma). This term also has traditionally included disorders such as irritable bowel syndrome (IBS) and noncardiac chest pain. For most of these disorders, treatment remains empirical and symptom based, reflecting ignorance of the pathophysiology involved.
PROKINETIC AGENTS AND OTHER STIMULANTS OF GI CONTRACTILITY
Prokinetic agents are medications that enhance coordinated GI motility and transit of material in the GI tract. These agents appear to enhance the release of excitatory neurotransmitter at the nerve-muscle junction without interfering with the normal physiological pattern and rhythm of motility. By contrast, activation of muscarinic receptors with the older cholinomimetic agents (see Chapter 9) or AChE inhibitors (see Chapter 10) enhances contractions in a relatively uncoordinated fashion that produces little or no net propulsive activity.
DOPAMINE RECEPTOR ANTAGONISTS
Dopamine (DA) is present in significant amounts in the GI tract and has several inhibitory effects on motility, including reduction of lower esophageal sphincter and intragastric pressures. These effects, which result from suppression of ACh release from myenteric motor neurons, are mediated by D2 dopaminergic receptors. DA receptor antagonists are effective as prokinetic agents; they have the additional advantage of relieving nausea and vomiting by antagonism of DA receptors in the chemoreceptor trigger zone. Examples are metoclopramide and domperidone.
METOCLOPRAMIDE. Metoclopramide (RSCEGLAN, others) and other substituted benzamides are derivatives of para-aminobenzoic acid and are structurally related to procainamide.
The mechanisms of action of metoclopramide are complex and involve 5HT4 receptor agonism, vagal and central 5HT3 antagonism, and possible sensitization of muscarinic receptors on smooth muscle, in addition to DA receptor antagonism. Administration of metoclopramide results in coordinated contractions that enhance transit. Its effects are confined largely to the upper digestive tract, where it increases lower esophageal sphincter tone and stimulates antral and small intestinal contractions. Metoclopramide has no clinically significant effects on large-bowel motility.
ADME. Metoclopramide is absorbed rapidly after oral ingestion, undergoes sulfation and glucuronide conjugation by the liver, and is excreted principally in the urine, with a t1/2 of 4-6 h. Peak concentrations occur within 1 h after a single oral dose; the duration of action is 1-2 h.
Therapeutic Use. Metoclopramide is indicated in symptomatic patients with gastroparesis, in whom it may cause modest improvements of gastric emptying. Metoclopramide injection is used as an adjunctive measure in medical or diagnostic procedures such as intestinal intubation or contrast radiography of the GI tract. Its greatest utility lies in its ability to ameliorate the nausea and vomiting that often accompany GI dysmotility syndromes. Metoclopramide is available in oral dosage forms (tablets and solution) and as a parenteral preparation for intravenous or intramuscular use. The initial regimen is 10 mg orally, 30 min before each meal and at bedtime. The onset of action is within 30-60 min. In patients with severe nausea, an initial dose of 10 mg can be given intramuscularly (onset of action 10-15 min) or intravenously (onset of action 1-3 min). For prevention of chemotherapy-induced emesis, metoclopramide can be given as an infusion of 1-2 mg/kg administered over at least 15 min, beginning 30 min before the chemotherapy is begun and repeated as needed every 2 h for 2 doses, then every 3 h for 3 doses.
Adverse Effects. The major side effects of metoclopramide include extrapyramidal effects. Dystonias, usually occurring acutely after intravenous administration, and parkinsonian-like symptoms that may occur several weeks after initiation of therapy generally respond to treatment with anticholinergic or antihistaminic drugs and reverse upon discontinuation of metoclopramide. Tardive dyskinesia also can occur with chronic treatment (months to years) and may be irreversible. Extrapyramidal effects appear to occur more commonly in children and young adults and at higher doses. Metoclopramide also can cause galactorrhea by blocking the inhibitory effect of dopamine on prolactin release (seen infrequently in clinical practice). Methemoglobinemia has been reported occasionally in premature and full-term neonates receiving metoclopramide.
DOMPERIDONE, A D2 RECEPTOR ANTAGONIST. In contrast to metoclopramide, domperidone predominantly antagonizes the D2 receptor without major involvement of other receptors.
Domperidone (MOTILIUM, others) is not available for use in the U.S. but is used elsewhere and has modest prokinetic activity in doses of 10-20 mg 3 times a day. Although it does not readily cross the blood-brain barrier to cause extrapyramidal side effects, domperidone exerts effects in the parts of the CNS that lack this barrier, such as those regulating emesis, temperature, and prolactin release. Domperidone does not appear to have any significant effects on lower GI motility.
SEROTONIN RECEPTOR AGONISTS
5HT plays an important role in the normal motor and secretory function of the gut (see Chapter 13). Indeed, >90% of the total 5HT in the body exists in the GI tract. The enterochromaffin cell produces most of this 5HT and rapidly releases 5HT in response to chemical and mechanical stimulation (e.g., food boluses; noxious agents such as cisplatin; certain microbial toxins; adrenergic, cholinergic, and purinergic receptor agonists). 5HT triggers the peristaltic reflex (see Figure 46–1) by stimulating intrinsic sensory neurons in the myenteric plexus (via 5HT1p and 5HT4 receptors), as well as extrinsic vagal and spinal sensory neurons (via 5HT3 receptors). Additionally, stimulation of submucosal intrinsic afferent neurons activates secretomotor reflexes resulting in epithelial secretion.
5HT receptors also are found on other neurons in the ENS, where they can be either stimulatory (5HT3 and 5HT4) or inhibitory (5HT1). In addition, serotonin also stimulates the release of other neurotransmitters. Thus, 5HT1 stimulation of the gastric fundus results in release of NO and reduces smooth muscle tone. 5HT4 stimulation of excitatory motor neurons enhances ACh release at the neuromuscular junction, and both 5HT3 and 5HT4 receptors facilitate interneuronal signaling. Developmentally, 5HT acts as a neurotrophic factor for enteric neurons via the 5HT2B and 5HT4 receptors. Reuptake of serotonin by enteric neurons and epithelium is mediated by the same transporter (SERT; see Chapters 5 and 13) as 5HT reuptake by serotonergic neurons in the CNS. This reuptake also is blocked by selective serotonin reuptake inhibitors (SSRIs; see Figure 15–1 and Table 15–1), which explains the common side effect of diarrhea that accompanies the use of these agents. Modulation of the multiple, complex, and sometimes opposing effects of 5HT on gut motor function has become a major target for drug development. The availability of serotonergic prokinetic drugs has in recent years been restricted because of serious adverse cardiac events. Tegaserod maleate (ZELNORM) has been discontinued; cisapride is available only via a restricted investigational drug protocol. A novel 5HT4 agonist, prucalopride (RESOLOR), is approved in Europe for symptomatic treatment of chronic constipation in women in whom laxatives fail to provide adequate relief.
CISAPRIDE. Cisapride (PROPULSID; Figure 46–2) is a 5HT4 agonist that stimulates adenylyl cyclase activity in neurons. It also has weak 5HT3 antagonistic properties and may directly stimulate smooth muscle. Cisapride was a commonly used prokinetic agent, however, it no longer is available in the U.S. because of its potential to induce serious and occasionally fatal cardiac arrhythmias that result from a prolonged QT interval. Cisapride is metabolized by CYP3A4 (see Chapter 6). Cisapride is contraindicated in patients with a history of prolonged QT interval, renal failure, ventricular arrhythmias, ischemic heart disease, congestive heart failure, respiratory failure, uncorrected electrolyte abnormalities, or concomitant medications known to prolong the QT interval. Cisapride is available only through an investigational, limited-access program for patients with GERD, gastroparesis, pseudo-obstruction, refractory severe chronic constipation, and neonatal enteral feeding intolerance who have failed all standard therapeutic modalities and who have undergone a thorough diagnostic evaluation, including an ECG.
Figure 46–2 Serotonergic agents modulating GI motility.
PRUCALOPRIDE. Prucalopride (RESELOR; see Figure 46–2) is a specific 5HT4 receptor agonist that facilitates cholinergic neurotransmission. It acts throughout the length of the intestine, increasing oral-cecal transit and colonic transit without affecting gastric emptying in healthy volunteers. Given in doses of 2 and 4 mg orally, once daily, the drug improves bowel habits. Prucalopride is approved in Europe for use in women with chronic constipation in whom laxatives fail to provide adequate relief.
MOTILIDES
MACROLIDES AND ERYTHROMYCIN. Motilin, a 22–amino acid peptide hormone found in the M cells and in some enterochromaffin cells of the upper small bowel, is a potent contractile agent of the upper GI tract. Motilin levels fluctuate in association with the migrating motor complex and appear to be responsible for the amplification, if not the actual induction, of phase III activity. In addition, motilin receptors are found on smooth muscle cells and enteric neurons.
The effects of motilin can be mimicked by erythromycin, a property shared to varying extents by other macrolide antibiotics (e.g., oleandomycin, azithromycin, and clarithromycin; see Chapter 55). In addition to its motilin-like effects, which are most pronounced at higher doses (250-500 mg), erythromycin at lower doses (e.g., 40-80 mg) also may act by other poorly defined mechanisms that may involve cholinergic facilitation. Erythromycin has multiple effects on upper GI motility, increasing lower esophageal pressure and stimulating gastric and small-bowel contractility. By contrast, it has little or no effect on colonic motility. At doses higher than 3 mg/kg, it can produce a spastic type of contraction in the small bowel, resulting in cramps, impairment of transit, and vomiting.
THERAPEUTIC USE. Erythromycin is used as a prokinetic agent in patients with diabetic gastroparesis, where it can improve gastric emptying in the short term. Erythromycin-stimulated gastric contractions can be intense and result in “dumping” of relatively undigested food into the small bowel. This potential disadvantage can be exploited clinically to clear the stomach of undigestible residue such as plastic tubes or bezoars. Rapid development of tolerance to erythromycin, possibly by downregulation of the motilin receptor, and antibiotic effects (undesirable in this context) limit the use of this drug as a prokinetic agent. A standard dose of erythromycin for gastric stimulation is 3 mg/kg intravenously or 200-250 mg orally every 8 h. For small-bowel stimulation, a smaller dose (e.g., 40 mg intravenously) may be more useful; higher doses may actually retard the motility. Concerns about toxicity, pseudomembranous colitis, and the induction of resistant strains of bacteria, among other things, limit the use of erythromycin to acute situations or in circumstances where patients are resistant to other medications.
Mitemcinal (GM-611), a macrolide non-antibiotic, shows promise for the treatment of gastroparesis.
MISCELLANEOUS AGENTS FOR STIMULATING MOTILITY
The hormone cholecystokinin (CCK) is released from the intestine in response to meals and delays gastric emptying, causes contraction of the gallbladder, stimulates pancreatic enzyme secretion, increases intestinal motility, and promotes satiety. The C-terminal octapeptide of CCK, sincalide (KINEVAC), is useful for stimulating the gallbladder and/or pancreas and for accelerating barium transit through the small bowel for diagnostic testing of these organs. Dexloxiglumide is a CCK1 (or CCK-A) receptor antagonist that can improve gastric emptying and has been investigated as a treatment for gastroparesis and for constipation-dominant IBS and may also have uses in feeding intolerance in critically ill individuals. Clonidine also has been reported to be of benefit in patients with gastroparesis. Octreotide acetate (SANDOSTATIN, others), a somatostatin analogue, also is used in some patients with intestinal dysmotility.
AGENTS THAT SUPPRESS MOTILITY
Smooth muscle relaxants such as organic nitrates and Ca2+ channel antagonists often produce temporary, if partial, relief of symptoms in motility disorders such as achalasia, in which the lower esophageal sphincter fails to relax, resulting in severe difficulty in swallowing. Preparations of botulinum toxin (BOTOX, DYSPORT, MYOBLOC), injected directly into the lower esophageal sphincter via an endoscope, in doses of 80-100 units, inhibit ACh release from nerve endings and can produce partial paralysis of the sphincter muscle, with significant improvements in symptoms and esophageal clearance.
LAXATIVES, CATHARTICS, AND THERAPY FOR CONSTIPATION
OVERVIEW OF GI WATER AND ELECTROLYTE FLUX. Water normally accounts for 70-85% of total stool weight. Net stool fluid content reflects a balance between luminal input (ingestion and secretion of water and electrolytes) and output (absorption) along the length of the GI tract. The daily challenge for the gut is to extract water, minerals, and nutrients from the luminal contents, leaving behind a manageable pool of fluid for proper expulsion of waste material via the process of defecation.
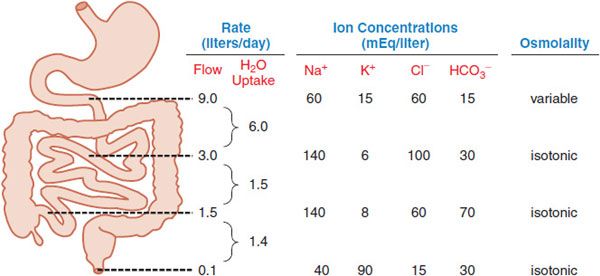
Normally ~8-9 L of fluid enter the small intestine daily from exogenous and endogenous sources (Figure 46–3). Net absorption of the water occurs in the small intestine in response to osmotic gradients that result from the uptake and secretion of ions and the absorption of nutrients (mainly sugars and amino acids), with only ~1-1.5 L crossing the ileocecal valve. The colon then extracts most of the remaining fluid, leaving ~100 mL of fecal water daily. Under normal circumstances, these quantities are within the range of the total absorptive capacity of the small bowel (~16 L) and colon (4-5 L). Neurohumoral mechanisms, pathogens, and drugs can alter secretion and absorption of fluid by the intestinal epithelium. Altered motility also contributes in a general way to this process. With decreased motility and excess fluid removal, feces can become inspissated and impacted, leading to constipation. When the capacity of the colon to absorb fluid is exceeded, diarrhea occurs.
Figure 46–3 The approximate volume and composition of fluid that traverses the small and large intestines daily. Of the 9 L of fluid typically presented to the small intestine each day, 2 L are from the diet and 7 L are from secretions (salivary, gastric, pancreatic, and biliary). The absorptive capacity of the colon is 4-5 L per day.
CONSTIPATION: GENERAL PRINCIPLES OF PATHOPHYSIOLOGY AND TREATMENT. Patients use the term constipation not only for decreased frequency, but also for difficulty in initiation or passage, passage of firm or small-volume feces, or a feeling of incomplete evacuation.
Constipation has many reversible or secondary causes, including lack of dietary fiber, drugs, hormonal disturbances, neurogenic disorders, and systemic illnesses. In most cases of chronic constipation, no specific cause is found. Up to 60% of patients presenting with constipation have normal colonic transit. These patients either have IBS or define constipation in terms other than stool frequency. In the rest, attempts usually are made to categorize the underlying pathophysiology either as a disorder of delayed colonic transit because of an underlying defect in colonic motility or, less commonly, as an isolated disorder of defecation or evacuation (outlet disorder) due to dysfunction of the neuromuscular apparatus of the rectoanal region.
Colonic motility is responsible for mixing luminal contents to promote absorption of water and moving them from proximal to distal segments by means of propulsive contractions. Mixing in the colon is accomplished in a way similar to that in the small bowel: by short- or long-duration, stationary (nonpropulsive) contractions. In any given patient, the predominant factor often is not obvious. Consequently, the pharmacological approach to constipation remains empirical and is based, in most cases, on nonspecific principles.
Constipation generally may be corrected by adherence to a fiber-rich (20-35 g daily) diet, adequate fluid intake, appropriate bowel habits and training, and avoidance of constipating drugs. Constipation related to medications can be corrected by use of alternative drugs where possible, or adjustment of dosage. If nonpharmacological measures alone are inadequate, they may be supplemented with bulk-forming agents or osmotic laxatives.
When stimulant laxatives are used, they should be administered at the lowest effective dosage and for the shortest period of time to avoid abuse. In addition to perpetuating dependence on drugs, the laxative habit may lead to excessive loss of water and electrolytes; secondary aldosteronism may occur if volume depletion is prominent. Steatorrhea, protein-losing enteropathy with hypoalbuminemia, and osteomalacia due to excessive loss of calcium in the stool have been reported. Laxatives frequently are employed before surgical, radiological, and endoscopic procedures where an empty colon is desirable. The terms laxatives, cathartics, purgatives, aperients, and evacuants often are used interchangeably. There is a distinction, however, between laxation (the evacuation of formed fecal material from the rectum) and catharsis (the evacuation of unformed, usually watery fecal material from the entire colon). Most of the commonly used agents promote laxation, but some are actually cathartics that act as laxatives at low doses.
Laxatives relieve constipation and promote evacuation of the bowel via:
• Enhancing retention of intraluminal fluid by hydrophilic or osmotic mechanisms
• Decreasing net absorption of fluid by effects on small- and large-bowel fluid and electrolyte transport
• Altering motility by either inhibiting segmenting (nonpropulsive) contractions or stimulating propulsive contractions
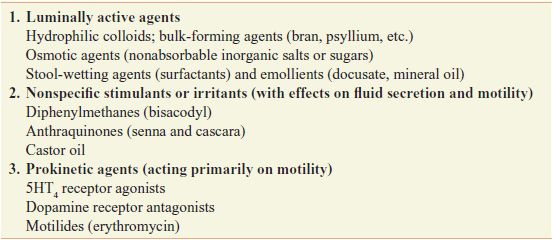
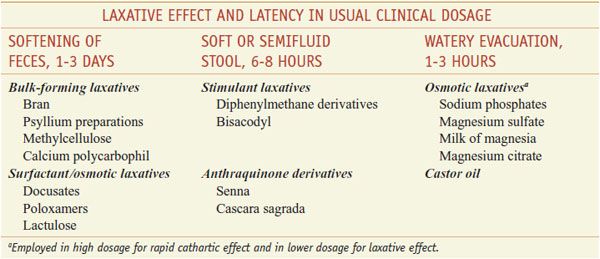
Laxatives can be classified based on their actions (Table 46–1) or by the pattern of effects produced by the usual clinical dosage (Table 46–2), with some overlap between classifications.
Table 46–1
Classification of Laxatives
Classification and Comparison of Representative Laxatives
A variety of laxatives, both osmotic agents and stimulants, increase the activity of NO synthase and the biosynthesis of platelet-activating factor in the gut. Platelet-activating factor is a phospholipid proinflammatory mediator that stimulates colonic secretion and GI motility. NO also may stimulate intestinal secretion and inhibit segmenting contractions in the colon, thereby promoting laxation. Agents that reduce the expression of NO synthase or its activity can prevent the laxative effects of castor oil, cascara, and bisacodyl (but not senna), as well as magnesium sulfate.
DIETARY FIBER AND SUPPLEMENTS
Bulk, softness, and hydration of feces depend on the fiber content of the diet. Fiber is defined as that part of food that resists enzymatic digestion and reaches the colon largely unchanged. Colonic bacteria ferment fiber to varying degrees, depending on its chemical nature and water solubility. Fermentation of fiber has 2 important effects: (1) it produces short-chain fatty acids that are trophic for colonic epithelium; (2) it increases bacterial mass. Although fermentation of fiber generally decreases stool water, short-chain fatty acids may have a prokinetic effect, and increased bacterial mass may contribute to increased stool volume. However, fiber that is not fermented can attract water and increase stool bulk. The net effect on bowel movement therefore varies with different compositions of dietary fiber (Table 46–3). In general, insoluble, poorly fermentable fibers, such as lignin, are most effective in increasing stool bulk and transit.
Table 46–3
Properties of Different Dietary Fibers
Bran, the residue left when flour is made from cereal grains, contains >40% dietary fiber. Wheat bran, with its high lignin content, is most effective at increasing stool weight. Fruits and vegetables contain more pectins and hemicelluloses, which are more readily fermentable and produce less effect on stool transit. Psyllium husk, derived from the seed of the plantago herb, is a component of many commercial products for constipation (METAMUCIL, others). Psyllium husk contains a hydrophilic mucilloid that undergoes significant fermentation in the colon, leading to an increase in colonic bacterial mass. The usual dose is 2.5-4 g (1-3 teaspoonfuls in 250 mL of fruit juice), titrated upward until the desired goal is reached. A variety of semisynthetic celluloses—e.g., methylcellulose (CITRUCEL, others) and the hydrophilic resin calcium polycarbophil (FIBER CSCON, FIBERALL, others), a polymer of acrylic acid resin—also are available. These poorly fermentable compounds absorb water and increase fecal bulk. Malt soup extract (MALTSUPEX, others), an extract of malt, is another orally administered bulk-forming agent. Bloating is the most common side effect of soluble fiber products (perhaps due to colonic fermentation), but it usually decreases with time.
OSMOTICALLY ACTIVE AGENTS
POLYETHYLENE GLYCOL–ELECTROLYTE SOLUTIONS. Long-chain polyethylene glycols (PEGs; MW ~3350 Da) are poorly absorbed and retain water via their high osmotic nature. When used in high volume, aqueous solutions of PEGs with electrolytes (COLYTE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree